Taking the Brakes Off Immune Cells to Treat Esophageal Cancer
The FDA approved the use of an immune checkpoint inhibitor to treat certain patients with a common form of esophageal cancer.
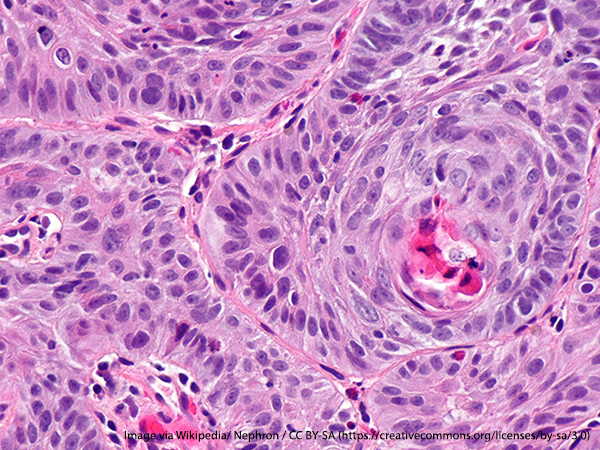
The U.S. Food and Drug Administration (FDA) granted approval for use of the immunotherapeutic nivolumab (Opdivo) in the treatment of patients with unresectable advanced, recurrent, or metastatic esophageal squamous cell carcinoma. The approval is indicated for patients who have previously received treatment with fluoropyrimidine-based or platinum-based chemotherapy.
Nivolumab is an immunotherapy treatment that targets a checkpoint molecule called PD-1, a protein on the surface of certain immune cells called T cells. The PD-1 protein helps to keep the immune system from overreacting and destroying healthy cells, but some cancer cells use this checkpoint system to evade immune detection. Nivolumab prevents the cancer cells from hijacking the PD-1 pathway so the T cells can identify and attack the cancer.
The data to investigate efficacy was compiled from a multicenter, randomized, active-controlled, open-label trial involving 419 participants whose disease progressed after previous treatment with the specified chemotherapy agents or who could not tolerate such treatment. The major efficacy outcome measure was overall survival among patients who received nivolumab versus those who received chemotherapy. Those who received nivolumab had a median overall survival of 10.9 months, compared with 8.4 months for participants in the chemotherapy arm of the study.
Esophageal squamous cell carcinoma is one of the two most common forms of esophageal cancer. As denoted by its name, it forms in the squamous cells—the thin, flat cells lining the epithelium of the esophagus. This cancer usually occurs in the upper and middle part of the esophagus, which is part of the upper gastrointestinal tract known as the food pipe because it moves food and liquid from the throat to the stomach. According to estimates by the National Cancer Institute, there will be more than 18,000 new cases of esophageal cancer in 2020 and over 16,000 deaths.
The FDA approval was rendered on June 10, 2020.
