November Editors’ Picks from AACR Journals
As we enter the winter holiday season, it’s time for the latest edition of Editors’ Picks, our monthly staple in which we highlight the “must read” articles chosen by the journal editors from each issue published by the American Association for Cancer Research. This month, selections range from a comparison of persistent opioid use following major surgery among patients with and without cancer, results from two clinical trials, and the preclinical development of an antibody-drug conjugate, among others. As always, studies featured here are freely available for a limited time.
Journal: Cancer Research (November 1 issue)
Pancreatic cancer is a lethal disease with limited response to several therapies, including immunotherapy. Due to the prevalence of epigenetic mutations in pancreatic cancers, epigenetic-modifying drugs have been explored as potential therapies. The authors previously found that the DNA hypomethylating drug decitabine altered methylation in tumor and stromal cells, inhibited tumor cell proliferation, and increased survival in a mouse model of pancreatic cancer. In this study, the authors explored the benefit of combining decitabine with immune checkpoint inhibitors against either PD-1 or V-domain Ig suppressor of T-cell activation (VISTA). They found that mice treated with single-agent decitabine had increased levels of tumor-infiltrating lymphocytes and M2-polarized macrophages, increased PD-1 expression, and increased tumor necrosis. While mice treated with single-agent decitabine or single-agent immune checkpoint inhibition had modest improvements in survival, treatment with decitabine followed by either immune checkpoint inhibitor led to an additive inhibition of tumor growth and prolonged survival. The greatest improvement in survival was observed in mice treated with decitabine followed by the PD-1 inhibitor, which extended mean survival from 32 to 54 days, compared to decitabine alone. The authors conclude that decitabine increases tumor infiltration of immune cells and significantly prolongs survival in mice when combined with immune checkpoint inhibition. A related commentary can be found here.
Journal: Cancer Discovery
An In Vivo Kras Allelic Series Reveals Distinct Phenotypes of Common Oncogenic Variants
KRAS is the most frequently mutated oncogene in human cancers, but the effects of KRAS mutation on tumor initiation, disease progression, or response to treatment remain unclear. Existing mouse models of mutated Kras do not represent the breadth of mutations that exist in human cancers. In this study, the authors utilized the CRISPR-Cas9 method of genome editing to generate new mouse models recapitulating six of the most common KRAS mutations seen in human cancers, all of which were at the G12 and G13 amino acid positions. Tissue-specific expression of each Kras mutant in the pancreas or colon of mice revealed differences in their ability to cause premalignant changes. The authors also developed pancreatic organoids that were derived from their mouse models and examined how different Kras mutants affected treatment response. They found that organoids expressing the Kras G13D mutant were sensitive to inhibition of the epidermal growth factor receptor (EGFR), and that organoids with the Kras G12C mutant were sensitive to G12C inhibitors only in the context of EGFR suppression. Together, the results indicate that various KRAS mutations may have diverse effects on cancer development and treatment response. This article was highlighted and featured on the cover of the November issue. A related commentary can be found here.
Journal: Clinical Cancer Research (November 15 issue)
For patients with multiple myeloma, standard-of-care treatment involves high-dose chemotherapy and autologous blood stem-cell transplantation (ASCT) followed by maintenance treatment with the antiangiogenesis agent lenalidomide (Revlimid), dosed at 10-15 mg per day. However, the impact of the maintenance dose has not been investigated, and side effects of lenalidomide commonly result in dose reductions. In this phase III clinical trial, the efficacy and tolerability of two extreme lenalidomide dose regimens, 25 mg and 5 mg per day, were investigated in patients with multiple myeloma who were treated with high-dose therapy and ASCT. After a median follow-up of 46.7 months, the median doses of lenalidomide in the high- and low-dose arm were 14.5 mg and 5 mg, respectively. The difference in progression-free survival (PFS) between the two arms was statistically significant, and the median PFS in the high- and low-dose arm was 44.8 and 33.0 months, respectively. Hematologic toxicity, severe neutropenia, and infections were more common in the high-dose arm but decreased after dose adjustments. Because lenalidomide dose correlated with efficacy and toxicity, the authors suggest a dose for each patient that equates to their current maximum tolerated dose, which is personalized through continuous up- and down-titration. This article was highlighted in the November 15 issue.
Journal: Cancer Prevention Research
Cirrhosis of the liver is a major risk factor for hepatocellular carcinoma (HCC). Patients with cirrhosis have an accumulation of neoantigens that may be targeted by immunotherapy. Here, the authors investigated the potential of immune checkpoint inhibition to prevent tumorigenesis in a mouse model of diethylnitrosamine and carbon tetrachloride-induced HCC. They found that treatment with anti-PD-1 therapy prior to tumor development led to a 46 percent decrease in the number of liver tumors, which was accompanied by an infiltration of CD4+ and CD8+ T cells into the liver. The authors did not observe increased liver dysfunction or worsening of overall health in mice that received anti-PD-1 therapy. The authors propose that immunotherapy may be a beneficial chemoprevention strategy for patients with cirrhosis who are at increased risk for HCC. This article was featured on the cover of the November issue. A related commentary can be found here.
Journal: Cancer Research (November 15 issue)
KRAS Controls Pancreatic Cancer Cell Lipid Metabolism And Invasive Potential Through the Lipase HSL
Cancer metastases, which account for 90 percent of cancer-related deaths, have different metabolic requirements than proliferative primary tumors and require additional energy for invasion. While obesity is an established risk factor for pancreatic cancer, how pancreatic ductal adenocarcinoma (PDAC) cells store and utilize fat to fuel metastasis has yet to be fully elucidated. In this study, the authors report that lipid droplets can drive PDAC migration and invasion in vitro. Further, the authors demonstrate that the oncogene KRAS controls the storage and utilization of lipid droplets through regulation of hormone sensitive lipase (HSL), an enzyme that the researchers report is downregulated in human samples of primary and metastatic pancreatic cancer. Further, disruption of the KRAS-HSL axis inhibited invasive migration in vitro and metastasis in vivo. These results identify a mechanism that could potentially be targeted to attenuate PDAC metastasis. This article was featured on the cover and was highlighted in the November 15 issue. A related commentary can be found here.
Journal: Molecular Cancer Research
Phosphorylation of PLCγ1 by EphA2 Receptor Tyrosine Kinase Promotes Tumor Growth in Lung Cancer
The receptor tyrosine kinase EphA2 is commonly overexpressed in non-small cell lung cancer (NSCLC) and is associated with poor clinical outcomes, representing an attractive therapeutic target. However, the underlying mechanisms that mediate EphA2 function, specifically its proximal downstream signals, have not been fully elucidated. In this study, the researchers performed a yeast two-hybrid screen and found that the protein phospholipase C gamma 1 (PLCγ1) interacts with EphA2, and that the kinase activity of EphA2 is required for the phosphorylation of PLCγ1. Genetic or pharmacologic inhibition of EphA2 in human lung cancer cells decreased the phosphorylation of PLCγ1. Additionally, loss of PLCγ1impaired tumor growth in a murine model of lung cancer. The authors conclude that the EphA2-PLCγ1 signaling axis promotes lung cancer growth, and that targeting this axis could be a promising therapeutic option for the treatment of lung cancer. This article was highlighted in the November issue.
Journal: Molecular Cancer Therapeutics
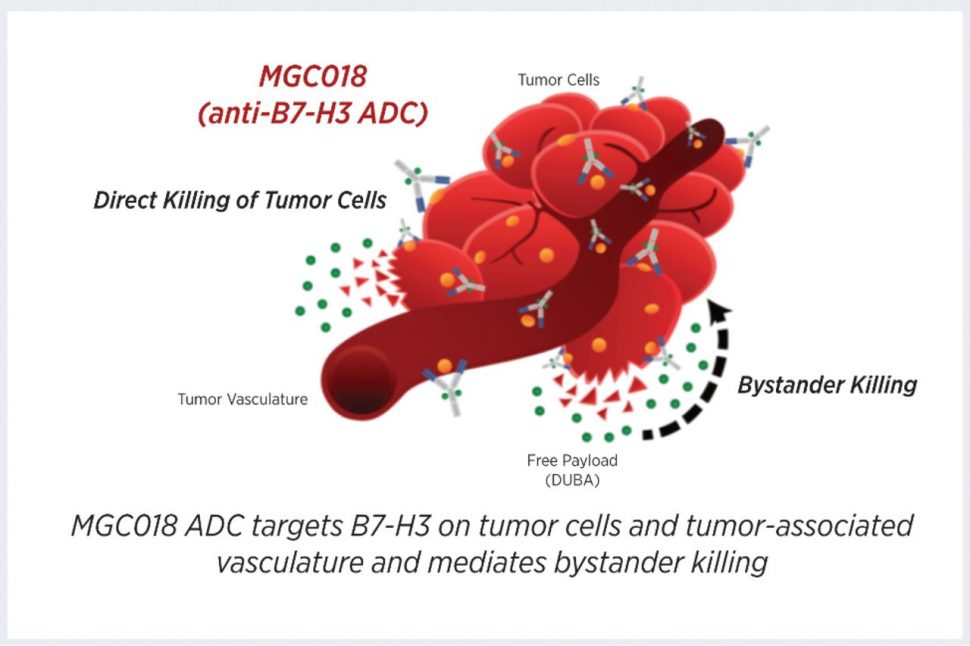
The transmembrane glycoprotein B7-H3 (also referred to as CD276) is an immune regulator that is overexpressed in many solid cancers but has limited protein expression in normal tissues, making it a potential therapeutic target for the treatment of solid tumors. This study describes the preclinical development of MGC018, an antibody-drug conjugate (ADC) with a duocarmycin payload that targets B7-H3. In vitro studies revealed that MGC018 was cytotoxic towards B7-H3-positive human tumor cell lines, and bystander killing of target-negative tumor cells was observed when co-cultured with B7-H3-positive cells. MGC018 treatment of preclinical models of breast, ovarian, and lung cancer, as well as melanoma, revealed potent antitumor activity. Finally, repeat-dose administration of MGC018 in cynomolgus monkeys resulted in a favorable pharmacokinetic and safety profile. The authors conclude that these results support the continued development of MGC018 for the treatment of solid cancers. This article was highlighted in the November issue.
Journal: Blood Cancer Discovery
The classification of tumors into molecular subgroups based on gene expression is used for many cancer types and helps identify patients at high risk, determine prognosis, and make treatment decisions. Several machine-learning protocols have been used to classify tumors into subgroups; however, existing methods require comparable training and testing datasets. In most cases, truly comparable datasets are not available, and testing datasets are instead normalized to an existing training cohort. This normalization can reduce the sensitivity or accuracy of the protocol. In this study, the authors modified their previously described classification method (named “probability ratio-based classification prediction score,” or PRPS) to allow for self-training of datasets. Using data from diffuse large B-cell lymphomas, they show that the revised method, called PRPS-ST, accurately classified tumors based on data from both RNA sequencing and hybridization-based methods. The authors propose that PRPS-ST is a useful tool for classifying tumors based on gene expression. This article was highlighted in the November issue.
Journal: Cancer Epidemiology, Biomarkers & Prevention
According to the Centers for Disease Control and Prevention, 128 people in the United States die every day as a result of an opioid overdose. Opioids are frequently prescribed for patients undergoing surgery to manage postoperative pain, yet patients with cancer may be especially vulnerable to persistent opioid use following surgery due to high levels of anxiety and depression, comorbid medical conditions, or the use of concomitant medications. To explore this potential association, the study authors conducted a retrospective analysis to evaluate persistent opioid use between patients with and without cancer following exposure to prescription opioids after surgery. The authors selected patients who underwent a hysterectomy or large bowel surgery, as both of these surgeries are prevalent and are performed for cancer and non-cancer indications. Among patients who underwent a hysterectomy, those who had cancer were more than twice as likely to develop persistent opioid use. Advanced cancer stage or receipt of systemic chemotherapy increased the risk of persistent opioid use in the cancer-hysterectomy cohort. However, cancer did not increase the risk of developing persistent opioid use among those who underwent large bowel surgery. The researchers conclude that additional research is necessary to evaluate optimal opioid prescribing strategies for patients with cancer. This article was highlighted in the November issue.
Journal: Clinical Cancer Research (November 1 issue)
Aberrant expression of histone deacetylases (HDAC) is associated with oncogenesis, and HDAC inhibitors have been approved for the treatment of certain cancers. Resistance to HDAC inhibition has been suggested to develop through activation of mTOR signaling, raising the possibility that combined inhibition of HDAC and mTOR may prevent resistance and improve responses. In this phase I clinical trial, the authors examined the clinical efficacy of the HDAC inhibitor vorinostat (Zolinza) in combination with the mTOR inhibitor sirolimus (Rapamune) or everolimus (Afinitor) in adult patients with heavily pretreated relapsed/refractory Hodgkin lymphoma, which is a population associated with poor clinical outcomes. The 22 patients in the vorinostat and sirolimus arm had an objective response rate of 55 percent and a median progression-free survival of 5.3 months. The objective response rate for the 18 patients in the vorinostat and everolimus arm was 33 percent, and patients in this arm had a median progression-free survival of 4.8 months. The most common grade 3 or 4 treatment-related adverse events for both arms were thrombocytopenia, neutropenia, and anemia. The authors propose that combined inhibition of HDAC and mTOR may improve clinical outcomes in patients with relapsed/refractory Hodgkin lymphoma. This article was highlighted in the November issue.
Journal: Cancer Immunology Research
The IL33 protein contributes to adaptive immunity by enhancing the function of T cells and can elicit antitumor effects when expressed in tumor cells or administered exogenously. However, the role of IL33 in the response to immune checkpoint blockade remains unclear. Using the MC-38 mouse model of colorectal cancer, the authors of this study examined whether treatment with immune checkpoint inhibitors targeting either CTLA-4 or PD-1 altered the expression of IL33 in tumor cells and the impact of these changes. Immune checkpoint blockade led to an increase in IL33 expression in tumor cells. By deleting the IL33 gene from tumor cells, the authors found that IL33 was required for the antitumor efficacy of immune checkpoint blockade. IL33 expression increased the number of T cells expressing the CD8 and CD103 proteins and of dendritic cells expressing CD103 in the tumor microenvironment. Administration of IL33 in combination with immune checkpoint blockade led to a synergistic increase in the survival of tumor-bearing mice. Together, the results indicate that IL33 contributes to immune checkpoint blockade therapy by recruiting specific immune-cell populations. This article was featured on the cover of the November issue.