Targeting a Rare Blood Cancer
The FDA approved a molecularly targeted therapeutic to treat certain pediatric patients and young adults with anaplastic large-cell lymphoma, a rare form of non-Hodgkin lymphoma.
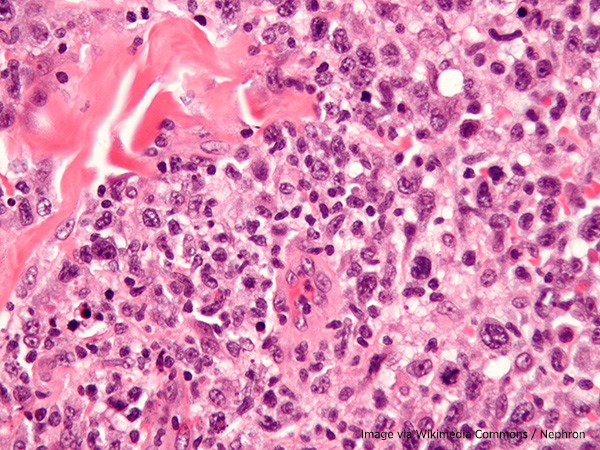
The U.S. Food and Drug Administration (FDA) has approved crizotinib (Xalkori) for pediatric patients over age 1 and young adults with relapsed or refractory systemic anaplastic large-cell lymphoma that tests positive for changes in a gene that makes a protein called anaplastic lymphoma kinase or ALK.
Anaplastic large-cell lymphoma is a form of non-Hodgkin lymphoma. Non-Hodgkin lymphoma involves abnormal growth of white blood cells, either T cells or B cells, that are part of the body’s immune system. Anaplastic large-cell lymphoma usually involves the T cells. It can show up either in the skin or other organs. It is considered aggressive when found in the lymph nodes or organs other than the skin.
In many children, anaplastic large-cell lymphoma is marked by a change in the structure of the ALK gene or by cells that make too much of the ALK protein. Cancer cells that have changes in the ALK gene or make too much ALK protein may grow more quickly and cancers that test positive for these abnormalities are called ALK-positive. Crizotinib belongs to a class of targeted therapeutics that block the action of the ALK protein.
Efficacy for the approval of crizotinib was evaluated in a multicenter, single-arm, open-label trial that included 26 patients who had received at least one prior systemic treatment. Patients in the study were in two groups, each of which received two different doses of crizotinib. The overall response rate was 88 percent, with a complete remission rate of 81 percent. Of the 23 patients who achieved a response, 39 percent maintained a response for at least six months and 22 percent maintained a response for at least 12 months.
Anaplastic large-cell lymphoma is one of the four major types of childhood non-Hodgkin lymphoma. According to the National Cancer Institute, there are about 800 new cases of non-Hodgkin lymphoma diagnosed in young people each year in the U.S., accounting for approximately 7 percent of cancers in patients under the age of 20.
The FDA decision was rendered on January 14, 2021.
