June Editors’ Picks from AACR Journals
Back for the month of June are the editors’ picks from the eight scientific journals published by the American Association for Cancer Research (AACR). Selections this month range from the identification of a long noncoding RNA involved in the canonical TGFβ/Smad signaling pathway to results from a clinical trial for patients with neuroendocrine tumors, a rare cancer type. Articles highlighted below are freely available for a limited time.
Journal: Clinical Cancer Research (June 15 issue)
The prognosis for patients diagnosed with neuroendocrine tumors (NETs) is often poor, and improved treatment options are urgently needed for this rare cancer type. In this phase Ib/II clinical trial, the antiangiogenic agent surufatinib (previously named sulfatinib) was evaluated in 81 patients with advanced NETs; 42 patients had pancreatic NETs and 39 had extrapancreatic NETs. Among patients with pancreatic NETs, the overall response rate (ORR), disease control rate, and progression-free survival (PFS) were 19 percent, 91 percent, and 21.2 months, respectively. Among patients with extrapancreatic NETs, the ORR, disease control rate, and PFS were 15 percent, 92 percent, and 13.4 months, respectively. Treatment-related adverse events of grade 3 or higher included hypertension (33 percent of patients), proteinuria (12 percent of patients), and hyperuricemia (10 percent of patients). The authors note that results from two ongoing phase III clinical trials evaluating surufatinib in patients with advanced pancreatic NETs and extrapancreatic NETs will provide even greater insight into the effectiveness of this investigational therapeutic as a treatment for NETs.
Journal: Cancer Research (June 15 issue)
Oncogenic KRAS, which has long been considered “undruggable,” fuels many human cancers. Identifying novel approaches to suppress oncogenic KRAS signaling represents an area of active investigation. In this study, the authors found that siRNA knockdown of Cnk1 (connector enhancer of kinase suppressor of Ras 1), which enhances Ras signaling pathways, inhibited the growth of lung and colon cancer cells harboring mutant KRAS. Molecular modeling studies helped to identify a compound, named PHT-7.3, that could selectively bind to the pleckstrin homology (PH) domain of Cnk1; administration of PHT-7.3 inhibited mutant KRAS cell and tumor growth in in vitro and in vivo studies. The authors conclude that targeting the PH domain of Cnk1 may represent a viable therapeutic strategy for KRAS-driven cancers.
Journal: Cancer Prevention Research
Vitamin D Signaling Suppresses Early Prostate Carcinogenesis in TgAPT121 Mice
Epidemiological studies assessing vitamin D levels and cancer risk have yielded inconsistent results, especially in prostate cancer. In this study, the authors evaluated how disruption of vitamin D signaling affects prostate cancer in mouse models. They found that genetic deletion of the vitamin D receptor enhanced prostate carcinogenesis. They also found that in dietary studies involving modification of vitamin D and calcium, which may suppress production of vitamin D, high levels of vitamin D reduced the incidence of adenocarcinoma of the prostate but did not improve bone mass, while high levels of calcium improved bone mass while increasing the incidence of adenocarcinoma of the prostate. These results suggest that modulation of vitamin D and calcium levels can influence bone mass and prostate carcinogenesis differentially.
Journal: Clinical Cancer Research (June 1 issue)
Mucosal-associated invariant T (MAIT) cells are a unique, innate-like subset of T cells that are enriched in the liver. Whether these cells have a role in hepatocellular carcinoma (HCC) is unknown. In this study, the authors evaluated the distribution, phenotype, function, and clinical significance of MAIT cells in patients with HCC. They found that HCC-infiltrating MAIT cells showed upregulation of the inhibitory molecules PD-1, CTLA-4, and TIM-3; reduced secretion of the proinflammatory cytokines IFNγ and IL17; and increased secretion of the tumor-promoting cytokine IL8. In addition, a high density of tumor-infiltrating MAIT cells was significantly associated with poor prognosis in patients with HCC. The authors conclude that the modulation of MAIT cells may provide a new therapeutic strategy for patients with HCC.
Journal: Cancer Epidemiology, Biomarkers & Prevention
While polyunsaturated fatty acids (PUFAs) have been shown to have a protective effect against cancer in animal models, studies evaluating whether they have a similar effect in humans have yielded mixed results. Following the identification of single nucleotide polymorphisms previously shown to influence levels of six PUFAs, the authors utilized Mendelian randomization to evaluate causal relationships between these PUFAs and human cancers among the UK Biobank cohort. None of the PUFAs studied had an association with overall cancer risk or mortality. Among individual cancers studied, higher levels of arachidonic acid were significantly associated with an increased risk for colorectal cancer. The authors state that future larger studies are needed to replicate the findings reported here for individual human cancers.
Journal: Cancer Research (June 1 issue)
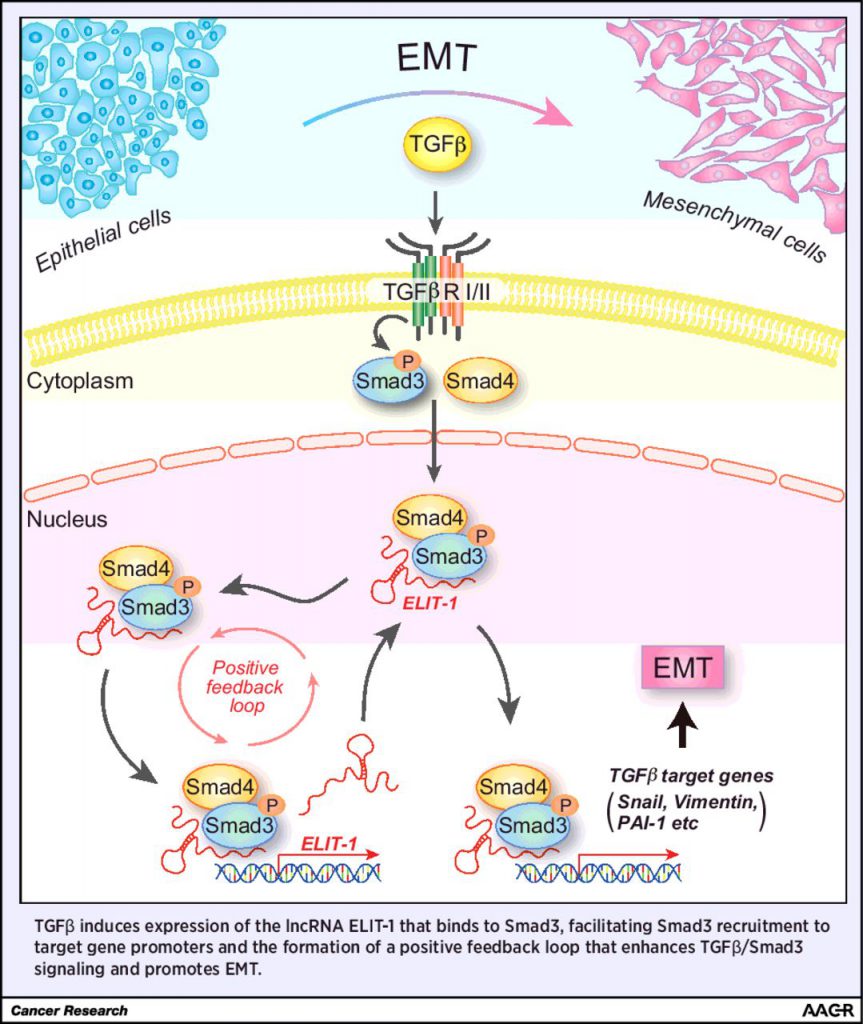
Transforming growth factor beta (TGFβ) can promote tumor progression by inducing epithelial-mesenchymal transition (EMT). Therefore, an active area of investigation is identifying approaches to inhibit TGFβ signaling pathways, such as the canonical TGFβ/Smad signaling pathway. In this study, the authors identified a novel cofactor of Smad3, the long noncoding RNA ELIT-1. Depletion of ELIT-1 using siRNAs inhibited TGFβ-mediated EMT and reduced expression of TGFβ target genes. Additionally, immunoprecipitation experiments revealed that ELIT-1 facilitates the recruitment of Smad3 to the promoters of target genes, such as Snail, vimentin, PAI-1, along with ELIT-1 itself. High expression of ELIT-1 was associated with poor prognosis in gastric cancers and lung adenocarcinomas, suggesting that this long noncoding RNA could serve as a prognostic biomarker for these cancers and as a potential therapeutic target.
Journal: Molecular Cancer Research
Prior studies suggest that the transcription factor TP63 influences the development and progression of head and neck squamous cell carcinoma (HNSCC), yet whether it functions as a tumor promoter or tumor repressor remains unclear. In this study, the authors found that the expression of TP63 was reduced in late-stage archival human HNSCC samples, and that genetic ablation of the Trp63 gene (the mouse homologue of the human TP63 gene) in head and neck epithelia increased the initiation and progression of HNSCC in mice. Loss of TP63 upregulated MAPK and STAT signaling and induced cervical lymph node metastases in human HNSCC xenografts. Furthermore, treatment with trametinib, a MEK inhibitor, abrogated metastases in the xenograft model. Taken together, these results suggest that targeting the MAPK signaling pathway may provide therapeutic benefit for HNSCC patients with low TP63 expression.
Journal: Molecular Cancer Therapeutics
Neutralization of BCL-2/XL Enhances the Cytotoxicity of T-DM1 In Vivo
Treatment with the antibody-drug conjugate T-DM1, while initially efficacious for many patients with advanced and/or HER2-positive metastatic breast cancer, often ultimately results in resistance. In this study, the authors investigated whether the inhibition of BCL-2/XL, which can mediate apoptosis, synergized with T-DM1 treatment in patient-derived breast cancer xenograft models. Two out of three models derived from advanced and treatment-exposed metastatic breast tumors displayed significantly enhanced T-DM1 cytotoxicity upon inhibition of BCL-2/XL, and a model derived from a treatment-naïve primary breast tumor showed sensitivity to T-DM1, which was enhanced upon combination treatment. The authors conclude that the combination of T-DM1 plus BCL-2/XL blockade warrants investigation in a future clinical trial.
Journal: Cancer Immunology Research
IL1R8 Deficiency Drives Autoimmunity-Associated Lymphoma Development
While patients harboring specific autoimmune or inflammatory conditions are more likely to develop lymphomas, the mechanistic link between autoimmunity and lymphoma development has not been fully elucidated. In this study, the authors monitored how deficiency of IL1R8, which negatively regulates inflammatory and autoimmune responses, affected lymphomagenesis in mice homozygous for the lymphoproliferation spontaneous mutation (lpr mice). They found that lpr mice with IL1R8 ablation developed diffuse large B-cell lymphoma (DLBCL), and that B cells isolated from such mice displayed constitutive activation of the NF-κB signaling pathway. Additionally, analysis of human DLBCL cells revealed that they express lower levels of IL1R8 compared with normal B cells, and that high expression of the IL1R8 gene correlated with a better prognosis, suggesting that downregulation of IL1R8 can help drive lymphomagenesis.
Journal: Cancer Discovery
While stimulation of the IL2 pathway has shown durable clinical benefit in a limited number of patients with certain types of cancer, treatment is often impeded by toxicity; instead of binding to the IL2Rα receptor, which is thought to contribute to adverse events, the IL2 pathway agonist NKTR-214 is biased to bind to the IL2Rβ receptor. This first-in-human phase I study evaluated the safety and early efficacy of NKTR-214 in 28 heavily pretreated patients with advanced or metastatic solid tumors enrolled across three centers. Of the 26 evaluable patients, none had an objective response, although 35 percent saw maximum tumor reductions that ranged from 2 to 30 percent according to RECIST. Analyses of peripheral blood and tumor biopsies following treatment with NKTR-214 revealed increased quantities of immune cells and enhanced immune activation. No adverse events above grade 3 were observed, and the recommended phase II dose was determined. As NKTR-214 enhances immune cell activity, it is also being studied in conjunction with other immuno-oncology agents, such as checkpoint inhibitors.