Elizabeth Buell-Fleming: A Childhood Reclaimed With Immunotherapy
Faced with a dramatically poor prognosis for their daughter, Elizabeth Buell-Fleming’s parents were pulled back from the cliff by an investigational monoclonal antibody.
There’s nothing typical about Elizabeth Buell-Fleming of Wilmington, Delaware.
Yes, like most 11-year-olds, she answers lots of questions from an adult with an “uh huh” or with short sentences that often end with “I don’t know.”
But Elizabeth is also a cancer survivor who, thanks to an investigational immunotherapy, overcame a poor prognosis for high-risk neuroblastoma that spread despite treatment including high-dose chemotherapy and a stem cell transplant.
Elizabeth’s journey began when she just 2 years old. It was the holiday season and her parents – Martha Buell and Boyd Fleming – decided to take her to the pediatrician to check on a cold she seemed to be fighting right before Christmas.
“She acted a little strangely when the doctor checked her abdomen,” Martha recalled. “The doctor said she felt something and that we should get an ultrasound just to be sure.”
But there didn’t seem to be any great urgency until Martha called the physician to say they’d made an appointment to have the ultrasound after the holidays. The pediatrician’s response was more pressing: “No, I think you should probably just go ahead and get it done today.”
That marked the start of Elizabeth’s – and her family’s – cancer journey.
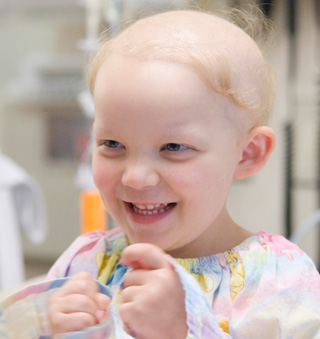
The little girl was diagnosed the day after Christmas in 2006.
“They called us as we were out just doing day-after-Christmas stuff and said, ‘She has a mass on her kidney. You have to get to the emergency room now.’ We just kind of dropped everything and ran and took her to the emergency room,” Martha recalled. “That was when we went from everything is really, really normal to everything is just crazy.”
At Nemours/Alfred I. duPont Hospital for Children, a scan revealed that Elizabeth had neuroblastoma, a cancer that originates in nerve tissue of the adrenal gland, neck, chest, or spinal cord and usually strikes children under 5 years of age.
Elizabeth had surgery in early 2007 to remove the grapefruit-sized tumor on her kidney. But then a lymph node removed during the operation showed signs of cancer. Further testing showed that the cancer had lots of copies of the MYC oncogene, meaning Elizabeth had the high-risk form of neuroblastoma.
Martha and Boyd took Elizabeth to Children’s Hospital of Philadelphia (CHOP) – which is known for its research and clinical care of kids with neuroblastoma – for a second opinion on the next steps for their daughter. Oncologists at CHOP recommended following their new treatment protocol for high-risk neuroblastoma.
The CHOP regimen meant that Elizabeth got six rounds of induction chemotherapy, a stem cell transplant, a course of high dose cis-retinoic acid, and radiation therapy directed toward the site of the tumor.
At that point Martha and Boyd sought to enroll Elizabeth in a clinical trial testing whether a new monoclonal antibody therapy called ch14.18 – a form of immunotherapy – after the treatment protocol for high-risk neuroblastoma would improve outcomes for these kids.
But to qualify for the clinical trial, the patient couldn’t have any evidence of cancer, so Elizabeth underwent a series of tests including an MIBG scan for neuroblastoma and a bone marrow biopsy.

“We were devastated when the results showed that there was cancer in Elizabeth’s bone marrow,” Boyd said. “Not only had the cancer appeared in a place where it had not been before, which meant her chance of survival had gone down dramatically, now she would no longer be part of the clinical trial.”
Martha and Boyd girded themselves for the worst. But Elizabeth’s physicians at CHOP did not give up. They advocated that she receive the investigational immunotherapy through a compassionate use exemption. The exemption was granted and Elizabeth became the first patient to get ch14.18 outside of the clinical trial.
Elizabeth’s treatment was completed in January 2010. She undergoes regular monitoring, which has found no evidence of disease.
“We are so grateful that cancer research made ch14.18 available just when Elizabeth needed it,” Martha said. “We were on the edge of a cliff and there will never be enough words to express the feeling that we felt when we were pulled back from the edge.”
On March 10, 2015, ch14.18 – called dinutuximab with the brand name Unituxin – was approved by the U.S. Food and Drug Administration “as part of first-line therapy for pediatric patients with high-risk neuroblastoma.
Read or download Elizabeth’s story in the AACR Cancer Progress Report 2015.