AACR Meeting Highlights Recent Advances in Pediatric Cancer Research
Pediatric cancer, while rare, is a devastating diagnosis that is estimated to affect over 11,000 children in the United States in 2019. Among those diagnosed between birth and age 14, more than 1,000 are anticipated to die from the disease this year. The most common types of cancer in this age group are leukemias, brain and other central nervous system (CNS) tumors, and lymphomas.
September is Pediatric Cancer Awareness Month, and it was therefore fitting that the American Association for Cancer Research (AACR) recently hosted the special conference, Advances in Pediatric Cancer Research, Sept. 17-20 in Montreal. This meeting, held in association with the AACR Pediatric Cancer Working Group, featured the most up-to-date findings on cancer predisposition and surveillance, targeted therapeutics, and immunotherapy in pediatric cancers.
Here we highlight several studies presented at the meeting that encompass key topics, including advances in pediatric surgery, precision medicine, biomarker research, and immunotherapy.
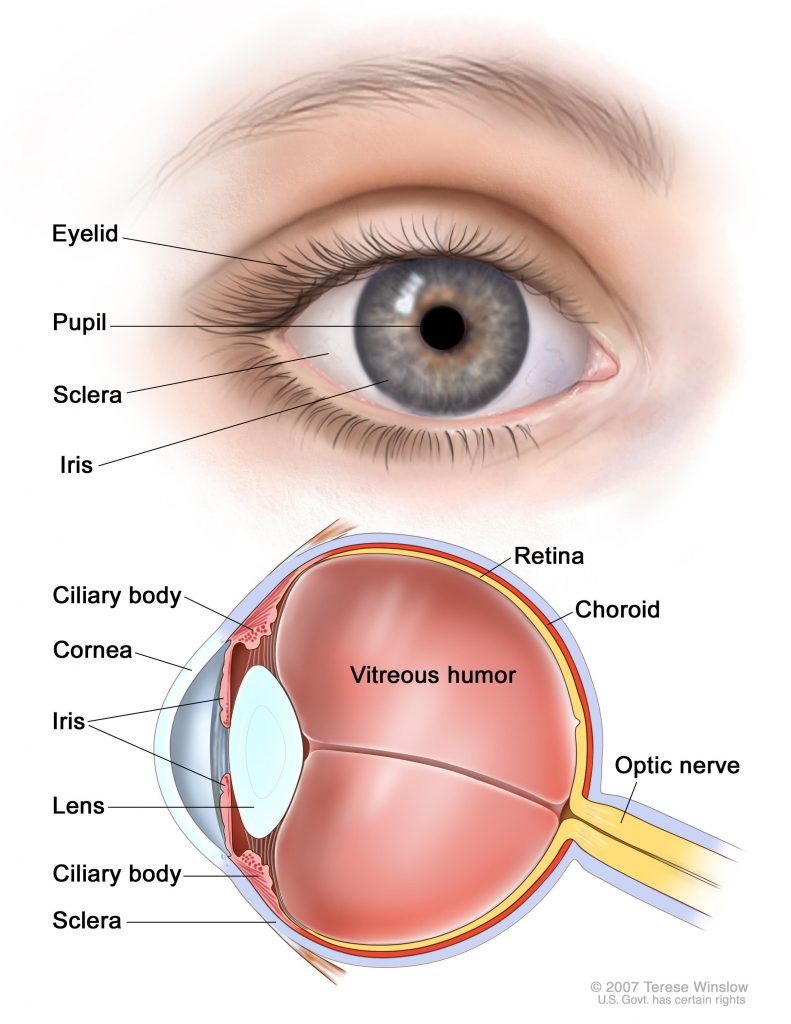
Pars plana vitrectomy for retinoblastoma
Almost exclusively a pediatric cancer, retinoblastoma is diagnosed in roughly 300 children and adolescents in the U.S. each year, and 95 percent of retinoblastoma cases are diagnosed before the age of 5. Treatment for the disease varies by tumor stage, and therapeutic options may include chemotherapy, radiotherapy, or cryotherapy. In cases where the tumor is large and vision is compromised, or if the cancer does not respond to other treatments, the eye may have to be surgically removed. While vision in the eye is lost, the removed eye is typically replaced with an orbital implant, which assists in subsequent bone and tissue growth and provides the foundation for an artificial eye.
A study presented in a poster session by Zhao Xun Feng from the University of Ottawa investigated the safety and efficacy of a more conservative approach – a tumor endoresection during a pars plana vitrectomy (PPV). In this procedure, part of the vitreous gel in the eye is removed, eliminating vitreous cancer cells and providing access to the retina, which may be resected. While this procedure can salvage the eye, this surgical treatment has not been widely adopted due to concerns of extraocular spread.
In this study, children were treated with PPV and endoresection for residual or recurrent retinoblastoma following chemotherapy. The researchers reviewed five-year outcomes for 227 children (comprising 247 eyes) treated at 29 Chinese centers between 2013 and 2014.
Among the 227 children, the five-year overall survival rate was 93.7 percent and the five-year eye-specific survival rate was 96.3 percent. Following this procedure, the five-year eye salvage rate was 80.7 percent. The authors conclude that the five-year eye-specific survival observed following PPV with endoresection is comparable to survivorship using other treatment modalities, and that this procedure has the potential to become the new standard of care in this setting.
Treatment decisions powered by a precision medicine platform
A study presented by Vanessa Tyrrell from the Children’s Cancer Institute in Sydney provided an update from the Precision Medicine for Children With Cancer (PRISM) trial, which is conducted under the Zero Childhood Cancer Program, a research initiative based in Australia. This multicenter prospective study, opened in September 2017, analyzes tumor DNA from children and adolescents under the age of 21 with high-risk cancer. The study combines results generated from comprehensive molecular profiling analyses with in vitro high-throughput drug screening and patient-derived xenograft drug efficacy testing, and a multidisciplinary board determines recommendations for a personalized treatment approach for these pediatric patients.
At the time of abstract submission, the trial had enrolled 213 patients; of these children, 36 percent had CNS tumors, 29 percent had sarcoma, 15 percent had leukemias/lymphomas, 7 percent had neuroblastoma, and 13 percent had other rare or unknown cancers.
The authors reported that the ZERO platform enabled at least one treatment recommendation for over 70 percent of the enrolled pediatric patients. Of the first 21 patients who received a recommended therapy that is not a standard of care for their respective cancers, 33 percent had a partial or complete response, 24 percent had stable disease, and 43 percent had progressive disease.
Utilization of genomic analyses to identify pathways to oncogenesis and new therapeutic targets was the focus of the fifth plenary session of this meeting.
Enhanced surveillance for children with therapy-related myeloid neoplasms
While cytotoxic therapies can have a variety of side effects, one potential consequence is the late development of therapy-related myeloid neoplasms (tMN), which are often extremely aggressive and can occur years after cancer treatment. These malignancies are typically resistant to conventional chemotherapies, often require hematopoietic stem cell transplantation, and carry a dismal prognosis. Identifying those with high-risk tMN is a critically unmet need, especially as the number of childhood cancer survivors continues to increase.
A study presented by Tamara Lamprecht from St. Jude Children’s Research Hospital evaluated RNA sequencing data from a cohort of 56 pediatric tMN cases. The researchers found that 43 percent of these patients had high levels of expression of the oncogene MECOM, corresponding to 24 pediatric patients. Patients with lower expression of MECOM had an overall two-year survival of 40.6 percent, while patients with higher expression of MECOM had an overall two-year survival of just 12.5 percent, suggesting that MECOM expression could be used as prognostic tool in pediatric patients with tMN.
Important aspects of pediatric cancer research, predisposition, and surveillance were the focus of a plenary session at the meeting.
Immunotherapy for embryonal brain tumors
Atypical teratoid rhabdoid tumors (ATRT) are a rare, aggressive type of CNS cancer that occur predominantly in children, with a five-year survival rate of around 30 percent. These tumors develop from aberrant embryonal cells and often spread to other areas of the CNS and to the spinal cord through cerebrospinal fluid. Most ATRT have alterations to the SMARCB1 gene, affecting the SWI/SNF complex, which is a key epigenetic regulator of gene expression.
In the second plenary session at the meeting, Johanna Theruvath from Stanford University presented a study detailing the preclinical investigation of a chimeric antigen receptor (CAR) T-cell therapy targeting B7-H3 (also known as CD276) in ATRT xenografts; the B7-H3 antigen was chosen due to its high expression in ATRT. The researchers evaluated if routes of CAR T-cell administration affected the efficacy, toxicity, and persistence of these CAR T cells.
Compared with intravenous CAR T-cell administration, the researchers found that locoregional intratumoral and intraventricular (ICV) administration of the CAR T cells had more potent antitumor effects. Following the clearance of ATRT, the researchers found that B7-H3 CAR T cells persisted in the brain and could protect from tumor re-challenge in the CNS or flank. The authors conclude that B7-H3 is a promising immune target for ATRT, and that delivery of B7-H3 CAR T cells to the CNS can mediate long-term protection from disease recurrence.
Immunotherapeutic approaches in pediatric cancers were also the focus of another plenary session at the meeting.
Looking forward
Due to increased awareness and technological advancements in the field, we have made substantial progress in finding new treatments for childhood cancers. Conferences such as this, which provide avenues for rapid information exchange and collaboration, are essential for the development of new treatments and breakthroughs for this rare collection of cancers.


Thanks for providing awareness and technologies of cancer treatment.