May Highlights from AACR Journals
This month’s selection of studies picked by the editors of the AACR journals includes a new bispecific antibody for treatment of solid tumors, a large-scale analysis of race-associated molecular changes in gynecologic malignancies, and a new method to characterize tumor evolution and response to therapy through genomic sequencing, among others. May also marks the debut of the latest addition to the AACR journal portfolio, Cancer Research Communications, in the Editors’ Picks feature.
Journal: Blood Cancer Discovery
Reconstructing Complex Cancer Evolutionary Histories from Multiple Bulk DNA Samples Using Pairtree
Understanding how cancers evolve can provide insight into cancer progression and inform treatment. Existing technologies are unable to capture the breadth of subclones present in cancer. Here, researchers describe the development and validation of Pairtree, a new method that can construct clone trees (models that describe evolutionary relationships between populations) containing upwards of 30 subclones. The method uses DNA-sequencing data from multiple samples of the same cancer to compile detailed clone trees. The researchers demonstrated that Pairtree was superior to existing clone tree development methods. In B-cell leukemias, Pairtree used up to 90 samples per cancer to reveal up to 26 distinct cell populations. The authors posit that Pairtree could be a valuable resource to understand the origin and progression of tumors, measure tumor age and heterogeneity, map mechanisms of treatment resistance, and understand the relationship between primary and metastatic tumors. This article is highlighted in the May issue. A related commentary can be found here.
Journal: Cancer Discovery
While immune checkpoint inhibitors targeting the PD-1/PD-L1 pathway have revolutionized treatment for certain cancers, they fail to elicit a sustained response against many tumors. Simultaneous engagement of 4-1BB, a T-cell costimulatory receptor found on certain activated immune cells, may enhance the antitumor effect of immune checkpoint inhibition; however, investigational monoclonal antibodies targeting 4-1BB have either had limited antitumor activity or unacceptable toxicity. In this study, the authors examined the preclinical and clinical efficacy of DuoBody-PD-L1x4-1BB (GEN1046), an investigational bispecific antibody that constitutively inhibits PD-L1 and conditionally stimulates 4-1BB. In cell culture models, GEN1046 led to greater T-cell proliferation, cytokine production, and antigen-specific cytotoxicity compared with clinically available PD-1/PD-L1 antibodies. Treatment with GEN1046 also led to tumor regression and longer progression-free survival in a mouse model of colon cancer. In a phase I/IIa clinical trial enrolling 61 heavily pretreated patients with advanced solid tumors, GEN1046 treatment led to increased levels of immune biomarkers that were consistent with its mode of action. At the time of data cutoff, 65.6 percent of patients experienced disease control, including four patients who had partial responses. The phase II portion of the trial is ongoing. This article is highlighted and featured on the cover of the May issue. A related commentary can be found here.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Neighborhood Obesogenic Environment and Risk of Prostate Cancer: The Multiethnic Cohort
Obesity has been suggested to be a risk factor for aggressive, high-grade prostate cancer. The authors of this study asked whether neighborhood factors linked to obesity impacted the risk of developing prostate cancer. To this end, they analyzed data from the California component of the Multiethnic Cohort; data from 43,223 participants who had not been diagnosed with prostate cancer prior to enrolling in the cohort were included in the analysis. The researchers found that participants who lived in neighborhoods with low socioeconomic status (SES) had a lower risk of nonaggressive prostate cancer compared with those who lived in neighborhoods with high SES. However, there were no significant associations between neighborhood SES and aggressive prostate cancer. Foreign-born Latino men who lived in neighborhoods with low mixed-land development had a greater risk of nonaggressive prostate cancer compared with those in areas with high mixed-land development. The associations between obesogenic neighborhood factors and prostate cancer risk were independent of the participants’ body-mass index, physical activity, or diet. The authors propose neighborhood obesogenic factors as important considerations when assessing prostate cancer risk. This article is highlighted in the May issue.
Journal: Cancer Immunology Research
Phosphoantigen-Stimulated γδ T Cells Suppress Natural Killer–Cell Responses to Missing-Self
Upon stimulation by phosphorylated compounds (referred to as phosphoantigens), γδ T cells contribute to an array of innate immune functions, including cytokine production, direct antitumor activity, and the initiation of adaptive immunity. Stimulation of γδ T cells by synthetic phosphoantigens has been explored as a potential therapeutic strategy for cancer but has thus far shown limited clinical efficacy. To better understand how phosphoantigen-stimulated γδ T cells might impact antitumor immunity, the authors of this study characterized the cytokine secretion profile of these cells and examined their effects on other immune cells. They found that phosphoantigen-stimulated γδ T cells had increased secretion of proinflammatory cytokines and reduced secretion of the anti-inflammatory cytokine IL-10 compared with unstimulated cells. Stimulation of γδ T cells suppressed the ability of natural killer cells to respond to cells lacking self-antigens (“missing-self response”) and to generate interferon γ, but it did not affect CD8+ T-cell responses or the ability of natural killer cells to mediate antibody-dependent cellular toxicity. Further analysis demonstrated that the suppressive effect on natural killer cells required direct interaction between natural killer cells and γδ T cells. The authors propose the suppression of natural killer cell responses to missing-self by γδ T cells as a potential reason for the limited clinical efficacy of synthetic phosphoantigens. A related commentary can be found here.
Journal: Cancer Prevention Research
Immunochemical fecal occult blood testing is an effective screening strategy for colorectal cancer, and it is recommended annually for individuals between ages 50 and 75. However, only a small percentage of individuals in Argentina are up to date with screening. In this study, researchers evaluated the efficacy of a workplace-based educational program for increasing screening awareness and uptake. The study population consisted of 1,552 individuals aged 50 or older, all employees of the Internal Revenue Agency of the Province of Buenos Aires (ARBA) and all of whom were insured through IOMA, the public health insurance provider for the province. The researchers performed a series of preintervention interviews and focus groups to determine appropriate intervention strategies and selected a multifaceted approach consisting of fliers (print and digital), emails, intranet and social media posting, and lectures and video messaging from executives. In the five months during and immediately following the intervention, testing rates were 16-fold higher than in the 51 months prior to the intervention—an increase from 8.4 tests to 130.5 tests per 100,000 individuals. The increase was highest in women aged 50 to 59. During the intervention period, the rates of screening remained stable for IOMA-insured individuals not employed by ARBA. The authors suggest that similar workplace-based intervention strategies could significantly increase colorectal cancer screening where it is most necessary.
Journal: Cancer Research (May 1 issue)
Melanoma cells are known to secrete factors that can prime distant tissues, such as lymph nodes, for metastasis. Fibroblastic reticular cells (FRC) help regulate contractility of lymph nodes, which can change the nodes’ architecture to facilitate or inhibit metastatic spread. Melanoma cells exist on a spectrum of cell states from melanocytic to dedifferentiated, and cells in these different states communicate with their microenvironment in different ways. In this study, the researchers sought to characterize the effects of different melanoma cell states on lymph node FRCs. Using human lymph node fibroblast cultures, the researchers found that secretion of the cytokine IL-1 by dedifferentiated cells (but not melanocytic cells) could inhibit JAK1-STAT3 signaling in FRCs, which decreased lymph node contractility by blocking the ROCK and YAP signaling pathways, thereby inhibiting actomyosin remodeling. Relaxed lymph node fibroblasts showed upregulated expression of many genes characteristic of fibroblast activation and cancer-associated fibroblasts. Conditioned media harvested from dedifferentiated melanoma cells and injected into mice decreased the stiffness of mouse lymph nodes and the contractility of lymph node fibroblasts. These data detail a mechanism by which melanoma cells promote metastasis by signaling to nearby lymph nodes and position blockade of IL-1 as a potential therapeutic strategy. A commentary on this study is available here.
Journal: Cancer Research (May 15 issue)
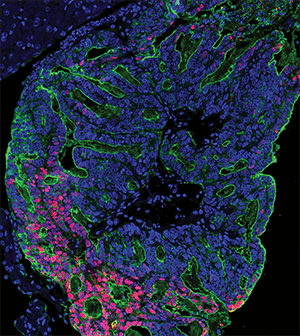
The main cause of colorectal cancer mortality is metastatic dissemination to distant organs, including liver and lungs. Micrometastases can remain dormant and occult for years after resection of the primary tumor and before actively growing lesions can be clinically detected. The authors of this study dissected the different stages of the transition from micrometastases to liver colonization in vivo, analyzing patient liver biopsies, patient-derived organoids, mouse models of colorectal cancer, and patient-derived xenografts. They found that liver micrometastases did not contain cancer stem cells (CSCs), but appearance of de novo CSCs through cellular plasticity correlated with growth and transition toward overt metastases. The cellular dynamics and transcriptional changes observed in vivo during metastasis formation were recapitulated in patient-derived colorectal cancer organoids, in which a heterogenous cellular composition correlated with continuous growth, underscoring the importance of cellular heterogeneity. The study revealed the pivotal and dynamic role of YAP signaling for the growth of colorectal cancer metastases: YAP activity was essential for proliferation of early-stage organoids but attenuation of YAP signaling was required to enable the formation of CSCs and sustained growth, while persistent YAP signaling led to a growth-stalled state and inhibited organoid maturation. This study was featured on the cover of the May 15 issue and related commentary is available here.
Journal: Clinical Cancer Research (May 1 issue)
The drug eribulin (Halaven) inhibits microtubule dynamics and has shown antitumor activity in a variety of solid tumor types, including treatment-refractory metastatic breast cancer, for which it is FDA-approved in the U.S. However, eribulin can cause significant hematological toxicities, which often limits dosing and decreases therapeutic efficacy. Packaging drugs in lipid spheres called liposomes can prevent premature degradation of the drug, limit uptake in off-target organs, and increase uptake in tumor tissue. In this phase I clinical trial, researchers evaluated the toxicity and preliminary efficacy of E7389-LF, a liposomal formulation of eribulin, in patients with advanced solid tumors. The study enrolled 21 patients in a “3+3” dose escalation design with doses ranging from 1 to 1.5 mg/m2 every two weeks or 1 to 2 mg/m2 every three weeks. The recommended dose for expansion was 2 mg/m2 every three weeks. The most common grade 3+ adverse events in this group were neutropenia and leukopenia, and three patients required a dose reduction. Among all dose levels, the clinical benefit rate was 66.7 percent, and the overall response rate was 19 percent, consisting of four partial responses. The authors suggest that these data warrant further investigation of liposomal eribulin for patients with solid tumors. This study was highlighted in the May 1 issue.
Journal: Clinical Cancer Research (May 15 issue)
IL-15 stimulates the proliferation and differentiation of natural killer cells and CD8 T effector memory cells and is considered a candidate cancer immunotherapy agent. However, IL-15 monotherapy was ineffective in a clinical trial on patients with metastatic cancer, likely due to the activity of immune checkpoints and a lack of specific tumor targeting, suggesting that combination strategies with other anticancer agents might be more effective. In this study, the authors evaluated the combination of IL-15 with anti-CD40 and anti-PD-1 treatment in mice bearing TRAMP-C2 prostate cancer cell tumors in both flanks. Anti-CD40 was included with the goal of stimulating intratumoral cytotoxic T lymphocytes, but it was administered intratumorally because parenteral administration was associated with unacceptable toxicity. Treatment with IL-15 or anti-CD40 alone resulted in partial inhibition of tumor growth and prolonged mouse survival; anti-CD40 also had a distal effect on the noninjected, contralateral flank tumors. Anti-CD40/IL-15 combination treatment provided additive inhibition of tumor growth associated with an increase in tumor-specific CD8 T cells and significantly improved survival. The addition of anti–PD-1 further improved the antitumor effect of the anti-CD40/IL-15 combination. According to the authors, these results support the clinical development of IL-15 in association with anti–PD-1 therapy and intratumoral anti-CD40. This article was highlighted in the May 15 issue.
Journal: Molecular Cancer Research
Modeling Androgen Deprivation Therapy-Induced Prostate Cancer Dormancy and its Clinical Implications
Between primary tumor treatment and disease recurrence, residual cancer cells often exist in a dormant state characterized by decreased growth and cell division. This pattern is common in prostate cancer treated with androgen deprivation therapy (ADT), a class of treatments that block protumor androgen signaling. Studying tumor dormancy has proven difficult, given that dormant lesions are often undetectable in patients. In this study, the researchers developed a set of patient-derived xenograft (PDX) mouse models of dormant prostate cancer in order to comprehensively profile dormant cells. A cohort of 55 mice was seeded with PDX tumors from 11 patients. After a period of tumor growth, some groups of mice were castrated and underwent tumor harvesting after 12 weeks (dormancy group), while others underwent long-term follow-up for assessment of recurrence. Molecular and transcriptomic analyses revealed two dominant subtypes of dormancy—one characterized by decreased cell proliferation and one characterized by a balance between cell growth and cell death. Both subtypes correlated with a poor prognosis compared to mice with no evidence of dormant disease, and both subtypes expressed biomarkers evident in the not-castrated “pretreatment” mice, which the researchers used to develop a 34-gene polygenic risk score to predict ADT response prior to therapy. These data contribute to an improved understanding of tumor dormancy and present possible opportunities for risk stratification of prostate cancer patients. This study was highlighted in the May issue.
Journal: Molecular Cancer Therapeutics
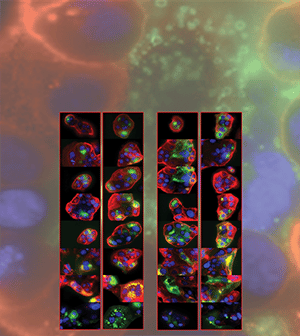
HER2-targeted monoclonal antibody therapies improve the survival of patients with HER2-positive breast cancer, but many patients develop progressive disease, therefore overcoming therapy resistance remains an unmet need. Approved small molecule inhibitors of HER2 also provide clinical benefit, but their use is limited by toxicities associated with off-target activity against other receptor tyrosine kinase proteins. Tucatinib (Tukysa) is an orally available, selective HER2 small molecule inhibitor that was approved for use in combination with the HER2 monoclonal antibody trastuzumab (Herceptin) and chemotherapy for patients with advanced metastatic HER2-positive breast cancer. In this study, the authors performed a comprehensive preclinical analysis of tucatinib as a single agent and in combination with other currently approved treatments. In vitro tucatinib treatment showed improved HER2 selectivity compared to small molecule HER2 inhibitors lapatinib (Tykerb) and neratinib (Nerlynx) in cell lines with HER2 amplification, including non-breast cancer cell lines and trastuzumab-resistant models. Tucatinib treatment induced tumor regressions in xenograft models of HER2-positive breast cancer, and combination of tucatinib and trastuzumab resulted in sustained blockade of HER2 downstream signaling. The efficacy of tucatinib combined with trastuzumab was comparable to that of the standard-of-care combination of trastuzumab, pertuzumab (Perjeta), and docetaxel, but was better tolerated and allowed for continuous dosing, supporting the use of tucatinib in chemotherapy-sparing combinations. Tucatinib also showed combined activity with CDK4/6 inhibitors in HER2-positive, estrogen receptor-positive xenograft models, indicating a potential therapeutic strategy for this difficult-to-treat subtype. According to the authors, these findings support expanded clinical development of tucatinib in combination with trastuzumab and other agents for HER2-driven cancers. This article was featured on the cover of the May issue. A related commentary is available here.
Journal: Cancer Research Communications
Race-associated Molecular Changes in Gynecologic Malignancies
Gynecologic cancer patients of African American (AA) descent endure worse outcomes than patients of European American (EA) ancestry. The authors of this study used The Cancer Genome Atlas (TCGA) and The Cancer Genome Ancestry Atlas (TCGAA), which allows for race-associated analysis of sequencing data, to characterize molecular changes potentially associated with the outcome disparities between AA and EA individuals. They performed a large-scale analysis of genetic and epigenetic alterations in individuals from AA and EA racial groups with malignancies of the breast, uterus, cervix, and ovary. This analysis identified novel molecular changes specific of AA tumors, including transcriptome alterations associated with malignant progression and metastasis, global upregulation of miRNA expression, and significant DNA methylation changes. Since epigenetic changes can be mediated by social and environmental factors including health care, food sources, and toxin exposure, the authors reasoned that their findings underscore the importance of taking an integrated approach to genomic analysis of racial cancer disparities, rather than studying the impact of individual genetic changes.



