A New Therapy for Bladder Cancer
The FDA granted accelerated approval to the targeted therapeutic sacituzumab govitecan to treat certain patients with the most common form of bladder cancer.
The U.S. Food and Drug Administration (FDA) recently granted accelerated approval to sacituzumab govitecan (Trodelvy) for patients with locally advanced or metastatic urothelial cancer, which is the most common type of bladder cancer. The indication is for patients who previously received a platinum-containing chemotherapy and a form of immunotherapy called an immune checkpoint inhibitor that targets the PD-1 protein on immune cells.
Sacituzumab govitecan is an antibody-drug conjugate designed to attach to a specific protein found on cancer cells and deliver a toxic agent to kill the cancer.
Efficacy and safety were evaluated in a single-arm, multicenter trial that enrolled 112 patients who had been previously treated with chemotherapy and an immune checkpoint inhibitor. The objective response rate to sacituzumab govitecan was 27.7 percent, with 5.4 percent demonstrating a complete response and 22.3 percent showing a partial response. The median duration of response was 7.2 months.
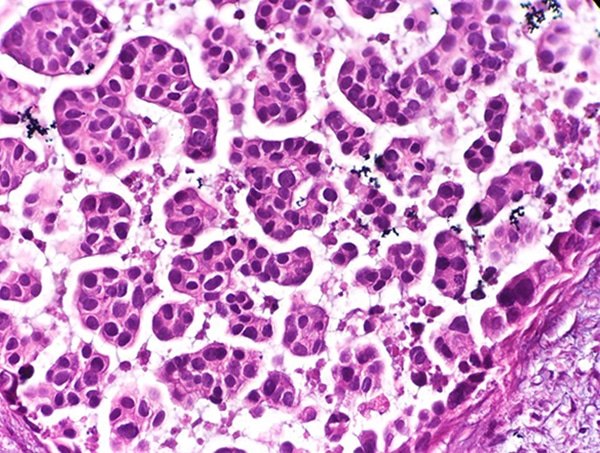
Urothelial cancer begins in cells found in the innermost layer of the bladder’s tissue. This tissue consists of multiple layers of epithelial cells, which can contract and expand to accommodate an empty or full bladder. According to federal statistics, there were an estimated 84,000 new cases of bladder cancer diagnosed in 2021, and men were more likely to be diagnosed with the disease than women.
The FDA granted accelerated approval to sacituzumab govitecan on April 13, 2021. Accelerated approval means continued approval may be contingent upon a confirmatory trial.
