A New Targeted Therapy for a Common Blood Cancer
The FDA has approved the targeted therapeutic loncastuximab tesirine-lpyl to treat certain adult patients with large B-cell lymphoma.
The U.S. Food and Drug Administration (FDA) has granted accelerated approval to loncastuximab tesirine-lpyl (Zynlonta) to treat adult patients with relapsed or refractory large B-cell lymphoma, which is the most common form of non-Hodgkin lymphoma. The indication is for patients who previously had two or more systemic treatments and includes patients with diffuse large B-cell lymphoma not otherwise specified as well as large B-cell lymphoma that arises from low-grade lymphoma and high-grade B-cell lymphoma.
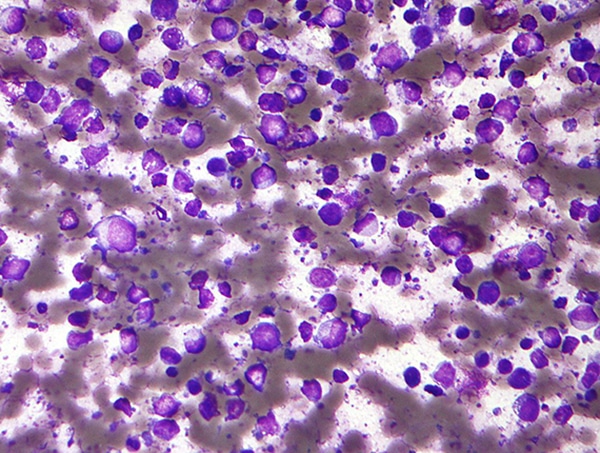
Loncastuximab tesirine-lpyl recognizes and locks on to a specific protein found on the surface of cancerous B cells, enabling the delivery of a toxic agent to kill the cancer cells.
The approval was based on an open-label, single-arm trial enrolling 145 adult patients who received the treatment until their disease progressed or they experienced unacceptable toxicity. The overall response rate was 48.3 percent, with 24.1 percent of the responders experiencing a complete response. After a median follow-up of 7.3 months, the median response duration was 10.3 months.
Diffuse large B-cell lymphoma is an aggressive cancer and as noted in its name, it develops in B cells—a type of white blood cell in the body’s immune system. This cancer often starts with a fast-growing mass in a lymph node under the arm or in the neck or chest area. This cancer can also develop in other parts of the body such as the intestines, bones, brain, or spinal cord.
According to federal statistics, in 2021 it was estimated that there would be more than 81,000 new cases of non-Hodgkin lymphoma diagnosed in the U.S. and diffuse large B-cell lymphoma would account for roughly 25-30 percent of them.
The FDA granted accelerated approval to loncastuximab tesirine-lpyl on April 23, 2021. Accelerated approval means continued approval may be contingent upon a confirmatory trial.
