AACR Annual Meeting 2018: Off-the-shelf CAR T-cell Immunotherapy – Are we There Yet?
A study presented at the AACR Annual Meeting 2018 discussed preliminary data on an off-the-shelf, T-cell receptor (TCR)-less, dual-targeted CD19-CAR T-cell product, FT819. In proof-of-concept preclinical studies, FT819 was found to be specific for and effective against CD19-positive cancer cells.
While the TCR is critical for T-cell generation and function, having a TCR on a CAR T cell has been implicated as one of the many setbacks in efforts to develop non-patient-derived CAR T-cell products. Therefore, the development of this TCR-less product that was functional in preclinical studies is a critical first step in bringing off-the-shelf CAR T-cell therapies to patients, the authors explain.
But what are off-the-shelf CAR T cells and why do we need them?
Chimeric antigen receptor, or CAR, T-cell therapy is a paradigm-shifting new treatment option for certain children and adults with blood cancers. The U.S. Food and Drug Administration approved two CAR T-cell therapies last year: tisagenlecleucel (Kymriah) was approved for treating certain pediatric and young adult patients with acute lymphoblastic leukemia (ALL), and axicabtagene ciloleucel (Yescarta) was approved for treating adults with certain types of non-Hodgkin lymphoma.
Treatment with CAR T-cell therapy, however, is a highly technical, complex process that can only be carried out at certain specialized centers. “The therapy is highly personalized, time-consuming to produce, and consists of only enough cells for a single-dose treatment with variable quality,” said Bob Valamehr, PhD, vice president of Cancer Immunotherapy at Fate Therapeutics Inc., who discussed the data on FT819 in a press conference. This process can also result in significant variability in quality, quantity, safety, and efficacy of the cells, he added.
A couple of research groups backed by pharmaceutical companies, including Fate Therapeutics and Allogene Therapeutics, are attempting to take CAR T-cell therapy to the next level. The goal is to develop an off-the-shelf CAR T-cell product that would not require personalizing the treatment for each individual patient.
The starting material to make off-the-shelf CAR T cells
The CAR T-cell product from Allogene Therapeutics, UCART19, was developed using genetically modified T cells from a healthy donor and engineered using TALEN, a gene-editing technology. This recent article in STAT discusses the development and progress made with UCART19. The FDA cleared the investigational new drug (IND) application for the clinical development of UCART19 in September 2017. A paper in Science Translational Medicine and this abstract from the American Society for Hematology conference in 2017 describe preliminary data from a clinical trial testing UCART19 in pediatric patients with relapsed/refractory B-cell acute lymphoblastic leukemia.
To make FT819, the researchers started with cells from a healthy donor to create a master cell line, and used the master cell line to produce large quantities of “universal” CAR19 T cells. These cells “can be packaged, stored, and made readily available for treatment of a large number of patients,” according to Valamehr.
The master cell line used to manufacture FT819 is an induced pluripotent stem cell (iPSC) line, which “provides distinct advantages over autologous (using cells from a patient’s own body) and allogeneic (using cells from a donor) approaches,” he said.
During the differentiation process, the master cell line is converted into CD34-positive cells and then differentiated into CD8-positive T cells.
A TCR-less product
To overcome the challenges with a serious, sometimes fatal condition called graft versus host disease (GvHD), the investigators developed a TCR-less product. The TCR is the portion of the T cell that enables the T cells to recognize pathogens and cancer cells as foreign and then act to eliminate them. These receptors are not compatible from patient to patient, so when donor T cells are given to a patient, they can attack the patient’s tissues and organs, resulting in GvHD.
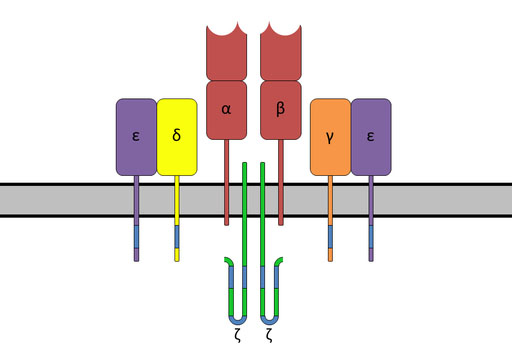
The T-cell receptor complex with TCR-α and TCR-β chains, CD3, and ζ-chain accessory molecules. Image via Wikipedia.
To make a TCR-less product, the researchers, along with Michel Sadelain, MD, PhD, a collaborator on the FT819 project at Memorial Sloan Kettering Cancer Center, used CRISPR/Cas9 gene-editing technology to direct the CAR to a T-cell receptor α constant (TRAC) locus. Sadelain had previously published a paper in Nature demonstrating that when CAR expression is under the control of a TRAC locus, it results in uniform CAR expression, enhances T-cell potency, and vastly outperforms conventionally generated CAR T cells in a mouse model of acute lymphoblastic leukemia.
In this review in Cell Stem Cell, Sadelain and colleagues discuss emerging T-cell engineering approaches using alternative T-cell sources, including TCR-less allogeneic T cells, expanded lymphoid progenitors, and iPSC-derived T cells.
Dual targeting may improve efficacy
Besides targeting CD19-positive tumor cells, FT819 has a second targeting receptor, the CD16 Fc receptor, designed to broaden the therapy’s efficacy, Valamehr said. This enables FT819 to be administered in combination with other proven cancer treatments, like monoclonal antibodies targeting CD20-positive tumor cells, to potentially overcome tumor antigen escape.
When challenged with CD19-positive tumor cells, FT819 mounted an efficient cytotoxic T-cell response, producing the cytokines IFN-gamma, TNF-alpha, and IL2 and the mediators of cell death, CD107a/b, perforin, and granzyme B. FT819 was also found to be target-specific, attacking only CD19-positive tumor cells and sparing CD19-negative tumor cells. Through the expression of the CD16 Fc receptor, FT819 was shown to elicit antibody-dependent cell-mediated cytotoxicity when combined with a therapeutic antibody targeting CD20.
“Through the development of FT819, we believe there is significant opportunity to lower the cost of CAR T-cell manufacture; enhance the quality of the product; and create a readily available supply of a more efficacious product to reach more patients in need,” Valamehr said. The investigators are preparing to file an investigational new drug (IND) application with the U.S. Food and Drug Administration for using the master iPSC line to develop their product.
A long way to go
While efforts from these labs to produce off-the-shelf CAR T-cell products appear promising, it is important to note that we are several steps removed from having an off-the-shelf product that patients can be treated with in a clinic. Given the potential of these approaches to make CAR T-cell therapies more accessible, it will be interesting to follow progress in this area.




