New Combination Therapy for Multiple Myeloma
The U.S. FDA approved a new combination of existing drugs to treat certain patients with multiple myeloma
The U.S. Food and Drug Administration (FDA) approved a new combination therapy for multiple myeloma in adult patients whose disease has come back after prior treatment or has stopped responding to treatment.
The new therapy includes a subcutaneous formulation of the anti-CD38 monoclonal antibody daratumumab combined with a protein called hyaluronidase (Darzalex Faspro) that helps it be injected under the skin and absorbed into the body; the proteasome inhibitor carfilzomib (Kyprolis); and the steroid dexamethasone.
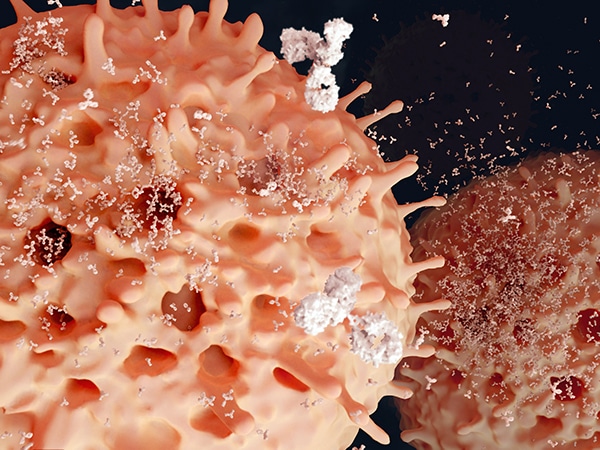
Anti-CD38 monoclonal antibodies are a type of targeted treatment used to kill multiple myeloma cells, which express the CD38 molecule on their surface. Proteasome inhibitors prevent myeloma cells from digesting and getting rid of excess proteins, causing a buildup that leads to cell death. Steroids, such as dexamethasone, help relieve pain and pressure by reducing swelling or inflammation in areas where myeloma cells accumulate and cause damage.
The approval of this combination was based on a single-arm cohort that involved 66 patients with relapsed or refractory multiple myeloma who had received at least one prior line of therapy as part of a multi-cohort, open-label clinical trial evaluating subcutaneous daratumumab in combination with other standard multiple myeloma treatments.
The overall response rate was 84.8 percent. The response endured for 6 and 9 months in approximately 85.2 percent and 82.5 percent of patients, respectively.
Multiple myeloma is a type of blood cancer and was expected to be diagnosed in nearly 35,000 people in the United States in 2021.
The FDA decision was rendered on November 30, 2021.
