A New Device to Monitor Heart Dysfunction in Childhood Cancer Survivors
Anthracyclines, a widely used class of chemotherapeutics, work in several ways to kill rapidly dividing cells, including those found in a tumor. While these drugs are commonly used to treat many types of adult and childhood cancer, they have a detrimental side effect – cardiotoxicity.
The cardiotoxicity of anthracyclines is dose-dependent; the more exposure patients have to the drug, the more serious risk they carry for heart-related problems. This can represent a unique challenge in children treated with anthracyclines, whose hearts are still developing.
“Initially the heart muscle may be able to compensate for the anthracycline-induced damage,” said Saro Armenian, a pediatric oncologist and director of the Childhood Cancer Survivorship Clinic at City of Hope in Duarte, California. “However, as survivors age and potentially develop health conditions such as diabetes or hypertension, this additional stress on the heart can result in an inability for the heart to pump effectively, which can manifest in heart failure.”
Indeed, childhood cancer survivors have high rates of cardiovascular complications. As reported in one study, childhood cancer survivors had nearly six times the risk for developing congestive heart failure compared to their siblings, and nearly five times the risk of having a myocardial infarction, or heart attack.
Many childhood cancer patients are treated with anthracycline-based chemotherapy, which can damage the heart. Photo by Bill Branson, courtesy of the National Cancer Institute.
How does treatment with anthracycline result in cardiotoxicity? The main theory is that cell death in the heart results in injury to the heart muscle fibers, explained Armenian. Due to this chemotherapy-induced injury, the wall of the heart muscle can get thinner and the heart chamber can enlarge, in a phenomenon known as dilated cardiomyopathy. This condition results in a decreased ability of the heart to pump blood throughout the body.
For cancer survivors treated with anthracyclines, the time frame for heart problems to manifest is extremely variable – issues can develop immediately following treatment, or they can occur 20 years later, Armenian noted. Because of this, members of the pediatric oncology community, such as the Children’s Oncology Group, recommend lifelong cardiac monitoring of these cancer survivors. For children treated with anthracyclines at a young age, lifelong monitoring of heart function can be burdensome, on many levels.
Moreover, it is estimated that less than 30 percent of long-term cancer survivors, defined as individuals surviving more than five years after their initial diagnosis, undergo routine risk-based screening, despite nearly 90 percent of these survivors receiving regular medical care. This lack of screening represents a clinically unmet need, and new methods to facilitate population-based screening are sorely needed, said Armenian.
Methods to measure heart dysfunction
Heart dysfunction is typically measured via echocardiography, which is essentially an ultrasound of the heart. This technique can measure the left ventricular ejection fraction (LVEF), a common metric used to assess heart function, which calculates the percentage of blood that is ejected from the left ventricle. An LVEF measurement of less than 50 percent may be an indication of impaired heart function, Armenian explained.
While echocardiography may be the standard of care for assessing heart dysfunction in childhood cancer survivors, this technique can yield variable results, noted Armenian. “LVEF measurements can vary from person to person, even if the heart function is the same between the two individuals, based on how the image is captured,” he explained. “Furthermore, echocardiography machines are available only at medical centers, they require a lot of space, and they can be fairly expensive. When we’re thinking about population-based screening, this technique is not necessarily ideal.”
The gold standard for measuring cardiac abnormalities is cardiac magnetic resonance imaging (MRI). Cardiac MRI can overcome many limitations associated with echocardiography – clinicians can generate accurate images of the heart and look at heart function and muscle health, among other things, noted Armenian. However, cardiac MRIs are extremely expensive, are often not available in community medical settings, and some patients are uncomfortable with this technique due to claustrophobia, he explained. As such, this device is also not tailored for population-based screening.
In a recent study published in Clinical Cancer Research, Armenian and colleagues analyzed the accuracy of Vivio, a prototype handheld device that collects pulse waves from the carotid artery to measure LVEF using a specialized algorithm. The data can be streamed wirelessly to a compatible device to monitor LVEF in real time and does not require image interpretation, Armenian explained. “This type of device could replace a screening echocardiogram for at-risk survivors of childhood cancer,” he noted. “One advantage of this device is its ability to stream the information to physicians, whether an oncologist or a general practitioner, in real time, anywhere. This could potentially help facilitate population-based screening for patients who are vulnerable for heart dysfunction.”
Assessing the accuracy of Vivio
Armenian and colleagues measured the LVEF in 191 childhood cancer survivors exposed to anthracyclines using Vivio, echocardiography, and cardiac MRI. The average LVEF measurement as analyzed via Vivio (56.8 percent) was similar to the average LVEF as measured by the gold standard cardiac MRI (56.5 percent). The average LVEF as measured by echocardiography, however, was high compared to cardiac MRI (61.7 percent), indicative of high false-negative rates.
Because Vivio uses an algorithm to determine LVEF, Armenian believes that measurements should be less variable than echocardiography. “The Vivio user will not be interpreting the images,” he explained. “The pulse wave is derived, an algorithm is applied, and the LVEF value is generated. This mobile health platform can thus help mitigate inter-observer variability.”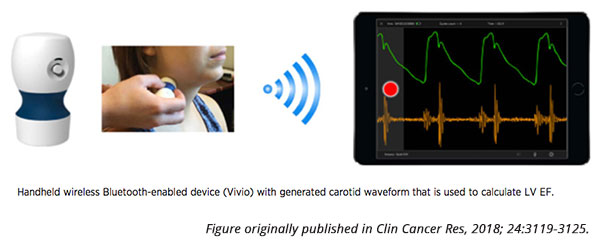
Furthermore, compared with cardiac MRI, Vivio had both a high sensitivity and a low false-negative rate when identifying patients with an abnormal LVEF (85.7 and 14.3 percent, respectively). “With Vivio, we didn’t miss many survivors who had abnormal heart function, which is very reassuring and is the ultimate goal in using a device like this for population-based screening,” said Armenian.
Importantly, Armenian does not see a device like Vivio replacing echocardiography or cardiac MRI. The main goal of Vivio, he explained, is to facilitate population-based screening. “One way to think about using this device is as a preliminary screening test,” Armenian explained. “If heart function is below a certain threshold as measured by Vivio, that patient can come back to the medical center for a more comprehensive assessment, as opposed to every single survivor coming back routinely for an in-depth echocardiogram.”
Incorporating telemedicine into survivorship care
“In addition to studying the burden of disease in childhood cancer survivors, it’s important to think about proactive approaches for surveillance and early detection to help potentially reverse heart disease in childhood cancer survivors before it becomes clinically apparent,” Armenian said. “In order to do that, we need to keep our survivors engaged, and one way to do that would be to integrate telemedicine in routine survivorship care for these patients. A device like Vivio could help bridge the inequities in survivorship care.”
Armenian noted that future studies with Vivio include determining its optimal use to facilitate large-scale screening. The device is still several steps away from becoming commercially viable, but this study demonstrating its accuracy was an important first step.