Editors’ Picks: November Highlights from the AACR Journals
With Thanksgiving behind us here in the United States, the holiday season is in full swing. So, grab some hot chocolate, cozy up, and read this month’s highlights from the 10 AACR journals, which include insights into the durability of immunotherapy responses, updated statistics on oropharyngeal and vulvar cancers, and the impact of African ancestry on triple-negative breast cancer.
As always, these articles will be freely available online for a limited time.
Journal: Blood Cancer Discovery
Responses to BCMA-targeted CAR T-cell therapies are typically short-lived in patients with myeloma, but the factors that influence response durability remain unknown. Here, researchers analyzed pre- and post-treatment bone marrow samples from patients with myeloma who had clinical responses to BCMA-targeted CAR T-cell therapy. They found that patients with long progression-free survival (PFS) (greater than six months) had increases in the proportion of T cells and decreases in the proportion of myeloid cells in the bone marrow following treatment. These changes were not observed among patients with short PFS (less than six months). The researchers also found that post-treatment CAR and non-CAR T cells from patients with long PFS had lower expression of immune checkpoint genes and other genes associated with T-cell exhaustion compared with those from patients with short PFS. Baseline features were also associated with PFS: Pre-treatment samples from patients with long PFS had greater T-cell receptor diversity, higher tumor expression of interferon response genes and mature plasma genes, and lower tumor expression of genes associated with epithelial-to-mesenchymal transition. “The major finding of this study is that the durability of response may be dependent on characteristics of non-CAR T cells and other immune cells in the tumor microenvironment,” said senior author Madhav Dhodapkar, MBBS, in an AACR press release. “This finding has broad implications for the CAR T-cell therapy field, as it emphasizes the importance of the patient’s preexisting immune microenvironment as a determinant of durable responses.” This article was highlighted in the November issue. A related commentary can be found here.
Journal: Cancer Discovery
Sub-Saharan African countries and women with African ancestry have high rates of triple-negative breast cancer (TNBC) and related mortality, raising the possibility that ancestry-associated genetic factors influence disease risk in women with African ancestry. In this study, researchers collaborated with the International Center for the Study of Breast Cancer Subtypes to perform RNA sequencing on tumor and non-tumor samples from African American, West African, and East African patients with TNBC to identify potential African ancestry-associated genetic factors. They found that the tumor expression patterns of 613 genes were associated with African ancestry. A subset of the identified genes also exhibited African ancestry-related expression patterns in normal breast tissue. Furthermore, analyses revealed that patients of African descent had distinct tumor-associated immune signatures. The authors suggest that the distinct gene expression profiles identified in this study could underlie the higher TNBC incidence and mortality among women with African ancestry. This article is highlighted and featured on the cover of the November issue. A related commentary can be found here.
Journal: Cancer Epidemiology, Biomarkers & Prevention
The Current Burden of Oropharyngeal Cancer: A Global Assessment Based on GLOBOCAN 2020
In this article, researchers reported the global burden of oropharyngeal cancer (OPC) using data from the 2020 version of GLOBOCAN. They estimated that 98,400 new cases of OPC were diagnosed in 2020, with 48,100 OPC-related deaths. This translated to an age-standardized global incidence of 1.1 per 100,000 individuals and an age-standardized global mortality rate of 0.51 per 100,000 individuals. These rates were about four-fold greater among men than women. Incidence was highest in Europe, North America, Australia, and New Zealand, whereas mortality was greatest in Central East Europe, Western Europe, Melanesia (a subregion of Oceania), South Central Asia, and the Caribbean. After accounting for differences in incidence, mortality rates were highest in North America, Australia, New Zealand, and North Europe and lowest in South Central Asia, most African regions, and Central America. The authors hypothesize that regional and gender-specific differences in tobacco/alcohol use and infection with human papillomavirus may contribute to the reported differences in OPC incidence and mortality. This article is highlighted in the November issue.
Journal: Cancer Immunology Research
The T-cell immunoglobulin and mucin domain protein 4 (TIM4) has been suggested to impact antitumor immunity in several different ways, depending on cell and tissue context. Here, researchers used immunolabeling and analysis of cancer transcriptome datasets to characterize TIM4 expression in tumors arising in different tissues and to examine its clinical significance. They identified two distinct populations of TIM4-expressing macrophages in the tumor microenvironments of patients with cancer: One was located within pleural and peritoneal cavities and the other was associated with tertiary lymphoid structures (TLS). Both macrophage populations also expressed the FOLR2 protein, which is a marker of tissue-resident macrophages that is associated with increased T-cell tumor infiltration. TLS macrophages positive for TIM4 and FOLR2 were associated with immune-reactive tumors and better prognosis, while pleural or peritoneal cavity macrophages expressing TIM4 and FOLR2 were associated with markers of immune suppression and worse prognosis. The findings suggest the presence of two distinct TIM4-positive macrophage populations with different tissue localization and contrasting roles in antitumor immunity. This article was featured on the cover of the November issue.
Journal: Cancer Prevention Research
Vulvar cancer is strongly associated with human papillomavirus (HPV) infections, with up to 80 percent of in situ cases linked to HPV. In 2006, the U.S. Food and Drug Administration (FDA) approved the first vaccine against HPV for routine use in adolescent girls, but trends in vulvar cancer incidence since then have not been comprehensively characterized. In this study, the researchers analyzed vulvar cancer incidence from 2000 to 2018, using the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results (SEER) databases, which together cover 98 percent of the U.S. population. The overall incidence of invasive vulvar cancer increased by an average of 1.3 percent per year; however, among women 20-44—the age group that may have been vaccinated—invasive vulvar cancer decreased by 0.8 percent per year. The incidence of invasive vulvar cancer also decreased by 0.6 percent per year among Hispanic women but increased by 1.7 and 1 percent among Black and non-Hispanic white women, respectively. The incidence of in situ vulvar cancer decreased by an average of 4.3 percent per year overall. Women aged 20-44 experienced the largest decrease in incidence, at an average of 6.5 percent per year. The researchers did not find a single time point where the slope of the decrease changed significantly, which suggests that factors beyond HPV vaccination may contribute to this decrease. The authors concluded that more time is likely required to understand the comprehensive impacts of HPV vaccination on vulvar cancer incidence.
Journal: Cancer Research (November 1 issue)
Genomic Landscapes and Hallmarks of Mutant RAS in Human Cancers
RAS genes are mutated in up to one-third of human cancers, but the prevalence of specific mutations differs based on the tissue of origin. In this study, the researchers sought to comprehensively characterize the associations between different RAS mutations and tumor site, patient characteristics, and other genetic mutations. Using RNA sequencing data from the AACR Project GENIE Registry, comprising 66,372 patient samples and representing 51 cancer types, the researchers confirmed that RAS mutations are most common in pancreatic cancer, colorectal cancer, lung cancer, and melanoma, and that they are least common in kidney cancer, breast cancer, and mesothelioma. The prevalence of total RAS mutations and of different RAS alleles differed not only by tissue of origin but also by the patient’s age, sex, and race. Mutations that commonly occurred alongside RAS mutations, as well as those that were mutually exclusive with RAS mutations, also differed based on the mutated allele and the tumor type; examples of common co-occurring mutations included NTRK3, PIK3CA, PTEN, and STK11, and common mutually exclusive mutations included BRAF and EGFR. The researchers expanded on these findings by evaluating multiomic analyses of 10,217 tumor samples from The Cancer Genome Atlas (TCGA). They associated different mutant RAS alleles with different altered signaling pathways, including cancer hallmarks like DNA repair, cell cycle checkpoints, and immune response, suggesting that different alleles may affect the tumor’s response to different forms of treatment. By analyzing survival data in the TCGA and GENIE cohorts, they found that the profile of mutations concurrent with each RAS allele could significantly impact a patient’s overall survival and response to immunotherapy. The authors suggested that understanding the physical, clinical, and molecular characteristics that coincide with each mutant allele could provide insights into the mechanisms underlying each mutation and thereby inform future therapeutic strategies. This article was featured on the cover of the November 1 issue.
Journal: Cancer Research (November 15 issue)
Research has shown that exercise can lower the risk of cancer recurrence, although the underlying mechanism is not fully elucidated. The authors of this study hypothesized that the metabolic reprogramming occurring in the organs due to exercise may limit the availability of nutrients in the tumor microenvironment, rendering it more resistant to metastatic colonization by cancer cells and creating a “metabolic shield.” The researchers tested this hypothesis using epidemiologic data and in vivo models of melanoma metastasis. Proteomic and metabolic analysis of internal organs from active and sedentary mice revealed a metabolic reprogramming towards increased energy production in lungs, lymph nodes, liver, and muscle from active mice. Similar metabolic changes were observed in routinely active persons following exercise. In addition, analysis of epidemiological data from a prospective cohort of 2,734 individuals who were cancer-free at the start of the study and were followed for 20 years revealed that high-impact exercise significantly reduced the risk of metastatic cancer.
In mouse models of melanoma, treadmill running prior to cancer injection significantly reduced metastatic spread to the lungs, lymph node, and liver compared with no exercise. In ex vivo experiments, inhibition of the mTOR pathway with rapamycin abolished the metabolic advantage of exercise, suggesting that this protective effect was dependent on mTOR activity. Finally, the authors found that the stroma from active mice consumed significantly more glucose than the tumor cells, whereas the opposite situation was observed in control mice. These findings suggest that exercise influences the metabolic landscape of the tumor microenvironment at the expense of the tumor and highlights the impact of exercise on cancer progression. This article was featured on the cover of the November 15 issue, and a related commentary is available here.
Journal: Clinical Cancer Research (November 1 issue)
The α-folate receptor (α-FR) is overexpressed in several tumor types, including ovarian cancer, and is not strongly expressed in healthy tissues. The authors of this study developed CT900—a cytotoxic thymidylate synthase inhibitor that enters the cell exclusively upon binding to α-FR—and sought to evaluate its safety and efficacy in patients with cancer in a phase I clinical trial. In the dose escalation phase of this trial, the researchers randomly assigned 42 patients to receive 1, 2, 4, or 6 mg/m2 of CT900 every week, or 2, 4, 8, or 12 mg/m2 of CT900 every two weeks. The 12 mg/m2 dose every two weeks was selected as the dosing schedule on which to base the expansion cohorts, to which the researchers exclusively recruited patients with high-grade serous ovarian cancer. The expansion cohorts included two treatment arms at 12 mg/m2 every two weeks—one arm with the steroid dexamethasone and the other without. Steroid inclusion did not significantly alter the adverse events profile or the overall response rate. The other expansion cohorts consisted of an arm at 12 mg/m2 every three weeks and 6 mg/m2 every two weeks, neither of which was analyzed independently. The overall response rate for the expansion cohort was 21.9 percent; when the cohort was analyzed based on α-FR expression, patients with high expression had an overall response rate of 36 percent, while those with low expression had an overall response rate of 7.7 percent. The researchers selected 12 mg/m2 every two weeks as the recommended phase II dose. This study was highlighted in the November issue.
Journal: Clinical Cancer Research (November 15 issue)
Robust biomarkers to predict response to immune checkpoint blockade are still lacking. The authors of this study have previously reported a correlation between response to anti-PD-1 therapy and the presence of a specific, tumor-reactive CD8+ tumor-infiltrating lymphocyte (TIL) population called PD-1T TILs, characterized by high expression of PD-1, in a small cohort of patients with non-small cell lung cancer (NSCLC), the most common type of lung cancer. In this article, the researchers conducted a retrospective study on two independent cohorts of NSCLC patients treated with anti-PD-1 therapy to validate the use of PD-1T TILs as a predictive biomarker and compare PD-1T TILs with other predictive markers. The frequency of PD-1T TILs in the tumor samples was assessed using an automated digital quantification workflow in 120 baseline samples before pembrolizumab or nivolumab treatment. PD-1T TILs predicted therapy response with 77 percent sensitivity (accuracy in identifying patients with disease control) and 67 percent specificity (accuracy in identifying patients with progressive disease) after six months of therapy, and with 93 percent sensitivity and 65 percent specificity after 12 months. A patient subgroup with PD-1T TILs below the identified threshold did not experience clinical benefit from treatment. Patients whose tumor tissue had high PD-1T TIL infiltration had significantly longer progression-free survival and overall survival than those with lower PD-1T TIL infiltration. Notably, in the same patient cohort, PD-1T TILs had a higher predictive performance than PD-L1 expression and tertiary lymphoid structures. According to the authors, these findings suggest that PD-1T TILs may impact treatment decision-making and help identify patients who are unlikely to benefit from PD-1 blockade. A commentary related to this study is available here.
Journal: Molecular Cancer Research
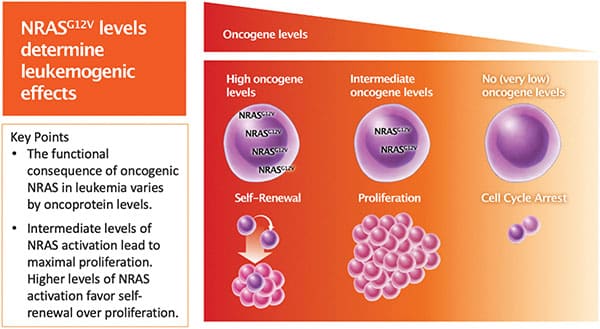
NRAS is frequently mutated in acute myeloid leukemia (AML), and the same NRAS mutation can drive mutually exclusive oncogenic effects. For example, AML cells with NRASG12V mutations are characterized by rapid proliferation or self-renewal, but rarely both. Previous studies have suggested that varying NRASG12V expression levels may explain the mutually exclusive effects on proliferation and self-renewal. In this study, the authors sought to test whether NRASG12V dosage mediates the mutually exclusive oncogenic effects and assess how downstream effectors of NRAS drive proliferation versus self-renewal. Using AML cell lines engineered to express dose-dependent levels of NRASG12V in response to doxycycline (DOX), the researchers confirmed that cells with higher expression of NRASG12V showed self-renewal characteristics, while cells with moderate NRASG12V expression were marked by rapid proliferation, and cells with little to no NRASG12V expression underwent cell cycle arrest. RNA sequencing and subsequent pathway analysis confirmed that signaling pathways involved in proliferation and self-renewal were enriched in cells with moderate and high NRASG12V expression, respectively. The researchers further assessed the expression of downstream NRAS effector proteins and found that the expression of some effectors—such as the proliferation drivers phospho-ERK, phospho-AKT, and phospho-4EBP1 and the self-renewal driver β-catenin—varied with the dose of NRASG12V. Other proteins, such as phospho-STAT1, phospho-STAT5, and p38, were consistently expressed regardless of NRASG12V dose. These data provide important insights into NRAS biology and could help inform future NRAS-targeting therapeutic strategies. This study was highlighted in the November issue.
Journal: Molecular Cancer Therapeutics
Anticancer Activity of ST101, A Novel Antagonist of CCAAT/Enhancer Binding Protein β
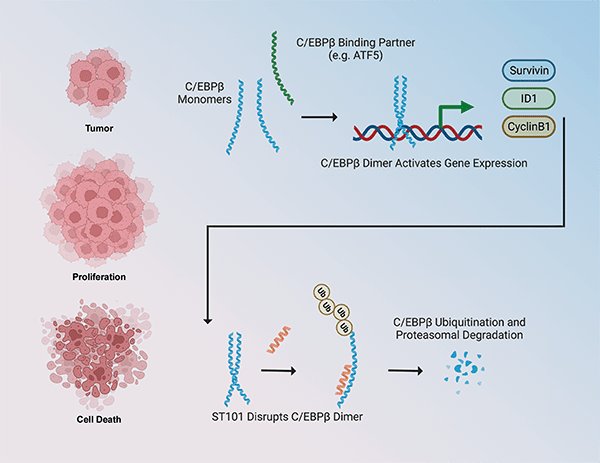
Overexpression or increased activation of the CCAAT/enhancer binding protein β (C/EBPβ) transcription factor is present in several cancer types, where it promotes cell proliferation, survival, and invasiveness and it correlates with worse prognosis and patient survival. In this study, the authors presented a novel selective antagonist of C/EBPβ that prevents its dimerization and favors its proteasomal degradation, therefore inhibiting its transcriptional activity and the activation of target signaling pathways. ST101 treatment caused cancer cell death in a panel of cancer cell lines including glioblastoma, breast, melanoma, prostate, and lung cancer, but not in normal cells. In mouse xenograft models, ST101 displayed potent antitumor activity both as a single agent and in combination with temozolomide. ST101 is currently being evaluated in a phase 1/2 clinical trial in patients with advanced solid tumors. According to the authors, the findings from this preclinical study support the clinical development of ST101 as a novel anticancer strategy targeting C/EBPβ, which was previously considered “undruggable”. This article was highlighted in the November issue and featured on the cover.
Journal: Cancer Research Communications
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer. PDAC is characterized by a dense stroma that is recognized as a mediator of disease progression, causing blood vessel compression and inducing significant metabolic adaptation in the tumor microenvironment to provide oxygen and nutrient supply to cancer cells. The main stromal components in PDAC are cancer-associated fibroblasts (CAFs) and the extracellular matrix they produce. This study focused on extracellular vesicles (EVs), which mediate the exchange of nutrients, proteins, and nucleic acids between CAFs and PDAC cells. The authors previously identified NetrinG1 (NetG1) as a driver of the tumor-supportive functions played by CAFs in PDAC. In this study, they found that NetG1-positive CAFs secrete small EVs (sEVs) that promote Akt-mediated survival pathways in tumor cells and protect them from apoptosis induced by nutrient deprivation. The researchers also showed that NetG1 expression in CAFs is necessary for sEV-mediated survival of PDAC cells. Further dissection of the CAF-derived sEVs showed two distinct fractions that differ in size and protein composition: exomeres, which range in size between 20 and 80 nm and contain NetG1, and exosomes, which range in size between 80 and 180 nm and contain the integrin α5β1 marker. Both NetG1 and integrin α5β1 were detected at higher level in sEVs isolated from the plasma of PDAC patients than in those obtained from healthy donors. According to the authors, this study elucidated a critical aspect of the tumor–stroma interaction in PDAC progression and suggested that EVs could be useful to study and monitor the PDAC tumor microenvironment.



