Targeting TNBC with an MDM2- PROTAC

Triple negative breast cancer (TNBC) is an aggressive cancer with high rates of p53 inactivation and lower survival rates than other breast cancer types due to increased metastasis and relapse (1). Owing to the frequent inactivation of p53, compounds that inhibit p53 from binding to its negative regulator, MDM2, are ineffective in TNBC. In a recent study published in Cancer Discovery, Dr. Eischen and her colleagues used an MDM2-targeted PROteolysis TArgeting Chimera (PROTAC) to reveal the requirement for MDM2 in p53 inactivated TNBC and identify a new therapeutic target for the disease (2)
Christine Eischen, PhD, an endowed professor of cancer research at Thomas Jefferson University, was awarded the AACR-Bayer Innovation and Discovery Grant in 2017 to investigate PROTACs targeting MDM2. She said, “The AACR-Bayer Innovation and Discovery Grant was awarded very early on in this project and provided financial support at a time in the project when other granting agencies would not have considered funding. Consequently, we were able to test several potential MDM2-PROTACs to identify a potential lead compound and begin to evaluate it at both a biochemical and biological level. These initial data allowed us to apply for and receive larger grants, and ultimately, a grant from the NCI that we were recently awarded that will enable Joseph Salvino, PhD (medicinal chemist at The Wistar Institute) and I to develop our MDM2-PROTAC for use in humans with p53-inactivated cancers.”
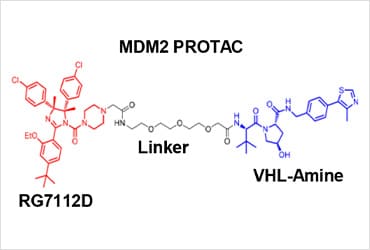
Dr. Eischen, in collaboration with Dr. Salvino and their research teams, designed and synthesized the MDM2-PROTAC, YX-02-030, by altering the clinically available MDM2 inhibitor, RG7112. PROTACs function by forming a ternary complex consisting of the target protein, the PROTAC and the E3 ubiquitin ligase complex to degrade the target protein. They replaced the 3-(methylsulfonyl)-propyl tail on the piperazine motif of RG7112 with an acetic acid to generate RG7112D and coupled this to VHL-Amine, the VHL E3 ligase recruiting ligand. The researchers then confirmed that the MDM2-PROTAC bound with a high affinity to MDM2 and VHL forming a ternary complex to induce selective degradation of MDM2.
doi:10.1158/2159-8290.CD-22-1131.
Investigating the biological effects of the MDM2-PROTAC, the lab then showed that the MDM2-PROTAC killed p53 wild-type and p53 inactivated TNBC cells in 2D and 3D cultures, patient TNBC explants, and xenografts in mice. Notably, they observed no signs of overt toxicity in the immune-competent or immune-deficient mice following MDM2-PROTAC treatment. Examining the lack of MDM2-PROTAC-induced toxicity further, the research team then tested normal CD34+ hematopoietic cells. They confirmed CD34+ cell viability and mRNA levels of p53 target genes (BAX, NOXA, PUMA) were unchanged following MDM2-PROTAC treatment, indicating the MDM2-PROTAC showed specificity to cancer cells.
Dr. Eischen and her colleagues then sought to understand how the MDM2-PROTAC, in contrast to MDM2 inhibitors, was capable of killing p53-mutant and p53-deleted TNBC cells. They first determined that the MDM2-PROTAC, but not MDM2 inhibitor, resulted in phosphorylation and stabilization of p53 family member p73 (TAp73); association with CBP/p300 acetyltransferase then led to transcriptional activation of TAp73. Evaluating RNA-sequencing data then revealed an increase in apoptotic genes (BAX, NOXA, PUMA, AEN, APAF1, TP5313 and PIDD1) after MDM2-PROTAC treatment. However, knockdown of TAp73 prevented this upregulation of apoptotic genes at the RNA and protein level. The researchers therefore concluded that the MDM2-PROTAC initiated an apoptotic mechanism mediated by TAp73 transcriptional up-regulation of pro-apoptotic genes.
Summarizing the main implications of the study, Dr. Eischen said, “We identified MDM2 as a new vulnerability in p53-inactivated triple negative breast cancer, in that it is required for continued survival of TNBC cells. The MDM2-PROTAC we designed and synthesized effectively targets MDM2 for degradation, causing TNBC cell death and revealing a new unexpected potential treatment avenue for TNBC patients.”
- Turner N, Moretti E, Siclari O, Migliaccio I, Santarpia L, D’Incalci M, et al. Cancer Treat Rev 2013;39:541-50. doi: 10.1016/j.ctrv.2012.12.001.
- Adams CM, Mitra R, Xiao Y, Michener P, Palazzo J, Chao A. Cancer Discov 2023; 13: 1210–1229. doi:10.1158/2159-8290.CD-22-1131