AACR Annual Meeting 2023: Boosting Diversity in Clinical Trials (Part 2, Community Engagement)
A study that examined the catchment areas of 102 cancer centers in the U.S. found that while over a third of U.S. counties are covered by two or more cancer centers, 15% of counties aren’t covered by any.
“The most disconcerting finding from this study may be the large number of Americans who are residing outside of a cancer center primary catchment area,” wrote the authors of the study, published in the AACR journal Cancer Epidemiology, Biomarkers & Prevention. “Undercoverage appears to be greatest in the Appalachian and Southern U.S., where cancer disparities have historically been large and continue to persist. Patients in these geographic areas are known to have poorly informed beliefs about cancer prevention and screening, are less likely to begin timely cancer treatment, and are less likely to enroll in a clinical trial.”
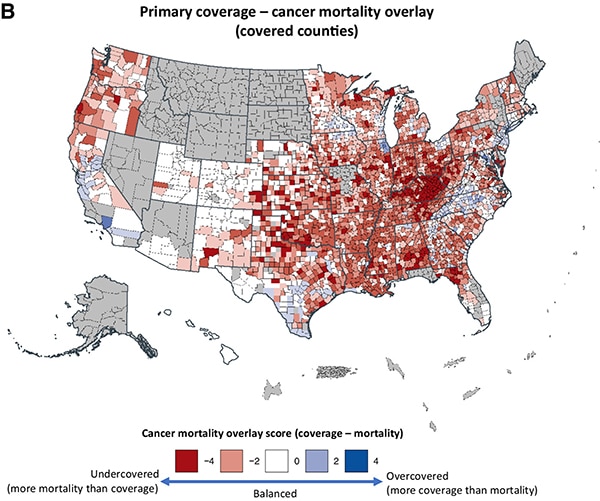
Such data highlight the limitations cancer centers may face in providing effective cancer care to underserved U.S. populations when working alone. Engaging community partners and local hospitals that serve geographically, racially, ethnically, and socioeconomically underrepresented populations is a key step to ensuring that the patients enrolled in clinical trials resemble the patients who will receive the new treatment.
Part one of this blog series featured presentations from the AACR Annual Meeting 2023, held April 14-19, focused on reshaping clinical trial design to facilitate the recruitment of patients from underserved communities into clinical trials. This second and final part will highlight effective community engagement strategies discussed at the meeting.
Best Practices for Community Engagement
At a forum titled “Beyond Platitudes and Proclamations: Meaningful Engagement of Diverse Communities in Cancer Research,” session chair Monica Baskin, PhD, a professor of medicine in the Division of Hematology/Oncology and associate director of health equity and community outreach and engagement at the University of Pittsburgh Hillman Cancer Center, explained that the process of community engagement is a cycle that begins with establishing credibility and trust. Subsequent steps, such as understanding the community context, communicating bidirectionally, and ensuring that individuals who participated in research see the results, feed forward to further strengthen credibility and trust.
Erica Warner, ScD, MPH, an assistant professor in the Department of Medicine at Harvard Medical School and an assistant investigator at Mass General Hospital, explained that massive breaches of trust, such as the Tuskegee syphilis study or the Puerto Rico Oral Contraceptive Trial, do not paint a full picture of the systemic injustices in clinical research.

“Sometimes when we think about history, it is a way in which we make these experiences seem distal—something that occurred in the past and is over. But, in fact, there are ongoing events, activities, and policies that continue to make us and our organizations potentially untrustworthy,” she said.
Such practices include transactional relationships, in which researchers only engage with the community to recruit clinical trial participants and fail to share the results from the trial with those communities; an influx of investigators with little experience in health equity research into the field due to its current popularity; and the failure to recognize a community research site as a full partner, sometimes leading to power imbalance and limited sharing of resources.
To ensure that interactions escape these pitfalls, Warner emphasized that researchers must be mindful of a community’s needs and critically evaluate whether their study will truly benefit the community. She also posited that trustworthiness should be measured and tracked over time, so researchers can evaluate what measures are and are not working.
Jiyoung Ahn, PhD, a professor of medicine and population health at the NYU Grossman School of Medicine, shared practices that helped shape her team’s engagement with the Asian American community.

The Food And Microbe Longitudinal Investigation (FAMiLI) study was initiated to study the link between gut microbiota and human disease in a population that is over 50% Asian American. Early in the study, Ahn and colleagues conducted a focus group and appointed a community advisory board that served as a liaison between researchers and the community and reviewed all plans and materials.
In terms of recruitment, Ahn and colleagues found that Asian Americans were especially receptive to the idea that their participation in clinical trials was a gift they could give to future generations, including their own children. The researchers also leveraged social media platforms popular with Asian Americans, such as WeChat and KakaoTalk.
For data collection, they translated and culturally adapted all surveys and instruction sheets to suit an Asian American population. Ahn and colleagues used short, versatile questionnaires that could be completed via text, email, web browser, or paper and could be saved to complete later if necessary. For sample collection, they used at-home collection kits with prepaid labeling for return to the lab.
“These principles could be applied broadly to many other racial and ethnic groups,” Ahn said.
Community Outreach in Action
The AACR Minorities in Cancer Research constituency group hosted a Scientific Symposium on Innovation to Address Disparities in Oncology Clinical Trials in which experts discussed institutional and government programs designed to foster engagement between cancer researchers and underrepresented communities.
Luis A. Diaz Jr., MD, FAACR, head of the Division of Solid Tumor Oncology in the Department of Medicine at Memorial Sloan Kettering Cancer Center and cochair of the session, began by outlining the stark injustices in how clinical research is performed and health care resources are allocated. “Despite this huge infrastructure—literally billions of dollars—to support the medical research cycle, there are tremendous disparities in clinical trial participation in underrepresented populations,” he said.

Folakemi Odedina, PhD, cochair of the session and a professor of internal medicine and associate director of the Center for Health Equity and Community Engagement Research at Mayo Clinic in Florida, discussed one solution she and her colleagues have spearheaded: “We have to make clinical trials an interaction.”
By implementing the Community Clinical Trials Champions Program, Mayo Clinic has forged relationships between their clinical trials office and churches, mosques, sororities, fraternities, American Legion posts, and other such organizations to educate members about clinical trials. During their outreach, they ask individuals if they would like to enroll in a community research registry to be notified in the future about clinical trials for which they may be eligible.
Odedina discussed other ways to bring clinical trials to more people, including setting up clinical trials offices or recruitment stations in heavily populated areas, in which anyone curious about clinical trials can drop by, ask questions, and enroll in the registry. She also mentioned mobile research units for collecting clinical samples to assist patients who cannot travel to the main research site.
The National Cancer Institute (NCI) has a similar suite of engagement programs, explained LeeAnn Bailey, PhD, MBBS, chief of the Integrated Networks Branch of the Center to Reduce Cancer Health Disparities at the NCI.

The Create Access to Targeted Cancer Therapy for Underserved Populations (CATCH-UP.2020) program granted awards to eight NCI-designated cancer centers with a proven track record of engaging minority populations to help recruit individuals from underrepresented groups into clinical trials testing targeted cancer drugs. The Connecting Underrepresented Populations to Clinical Trials (CUSP2CT) program similarly funds studies to test culturally tailored recruitment strategies to refer more patients from underrepresented racial and ethnic groups into clinical trials.
“Without referral, without engagement, you can’t affect enrollment or recruitment downstream,” Bailey said.
Bailey also discussed the National Community Oncology Research Program (NCORP), which conducts clinical trials related to screening, prevention, surveillance, supportive care, and health-related quality of life. The program’s stated goal is to “bring cancer clinical trials and care delivery studies to people in their own communities.” Through their network of 46 community sites, 14 of which serve at least 30% underrepresented minorities, they recruit diverse patients to clinical trials run by a variety of NCI programs.
The U.S. Food and Drug Administration (FDA) hosts programs to foster the inclusion of underserved patients beyond racial and ethnic minorities, said Jamie Brewer, MD, a medical oncologist and acting Clinical Team Lead in the Office of Oncologic Diseases at the FDA.
“Diversity should be considered across demographic characteristics such as race, ethnicity, age, geography, and across clinical characteristics that may impact outcomes or responses to therapy, such as hepatic or kidney dysfunction,” Brewer explained.

Brewer mentioned Project Silver, which aims to boost the enrollment of cancer patients aged 65 and older, who are often excluded from cancer clinical trials. Brewer also discussed Project Community, which caters to the needs of cancer patients, survivors, and advocates living in rural areas.
“It is entirely focused on relationships—building relationships, providing information to the community and the populations affected by cancer,” Brewer said. “We really want with Project Community to establish long-term connections and a channel for communication and outreach.”
For more information from the Annual Meeting that involves health disparities and the importance of diversity, you can browse the sessions selected for the Cancer Disparities track here. Recordings for each session will be available to registered meeting attendees on the virtual conference platform through July 19, 2023.
Additionally, abstract submission is now open for the 16th AACR Conference on the Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved, with registration available soon.



