FDA Approvals in Oncology: July-September 2023
With the approval of new anticancer therapeutics, more treatment options become available for patients. Some therapies are new to the market, while some may have already been approved for other indications; some molecules are first in class, directed against a previously untargeted pathway or acting through a new mechanism, while others may be improved versions of drugs that already exist.
To help our readers keep track of the cancer therapies approved by the U.S. Food and Drug Administration (FDA), understand their impact for patients, and put them in context of the current therapeutic landscape, Cancer Research Catalyst provides a quarterly review of the latest approvals from the FDA.
Approvals issued in July, August, and September 2023 included a new treatment for prostate cancer harboring BRCA mutations, two new bispecific T-cell engagers for multiple myeloma, a kit to treat liver metastases from uveal melanoma, and more.
Advances in Targeted Therapies
Targeted therapies, designed to inhibit a specific cancer driver protein, form a key pillar of cancer therapy. In tumors harboring a gene that has been mutated, rearranged, or overexpressed, targeted therapies can home in on the abnormality and block its cancer-promoting signals, often resulting in cell death.
Tyrosine kinase inhibitors (TKIs) are a common class of targeted therapies. These small molecules inhibit tyrosine kinases—proteins that relay signals inside a cell. While tyrosine kinase activity is tightly regulated, mutations and other alterations can override the regulatory mechanisms and stimulate aberrant signaling, leading to cell proliferation.
This quarter, three TKIs were approved by the FDA for new indications.
- In July, the FDA approved quizartinib (Vanflyta) for use during multiple stages of treatment in patients with newly diagnosed acute myeloid leukemia (AML). Quizartinib targets the kinase FLT3, which is mutated in around one-third of AML cases. The most common FLT3 mutation in AML is an internal tandem duplication, in which parts of the FLT3 gene are copied several times alongside each other.
AML treatment typically occurs in three stages: induction, in which chemotherapy eradicates most leukemia cells; consolidation, in which chemotherapy kills any remaining cancer cells; and maintenance, consisting of treatment to prevent a recurrence. For patients with a FLT3 internal tandem duplication, quizartinib can now be added to induction and consolidation regimens and can be used as a single therapy for maintenance.
- In August, the FDA granted full approval to pralsetinib (Gavreto) for patients with metastatic non-small cell lung cancer (NSCLC) in which the RET gene has fused with another gene. The fusion may disrupt RET’s normal regulatory mechanisms and may cause it to be expressed at places and times that aren’t typical. The FDA previously granted pralsetinib an accelerated approval for this indication in 2020.
Accelerated approval indicates that continued approval may be contingent upon additional confirmatory trials, the results of which are submitted to the FDA as part of an application for conversion to full approval.
- In September, the FDA approved bosutinib (Bosulif) for certain pediatric patients with chronic myelogenous leukemia (CML). Many CML cases are driven by the BCR-ABL kinase, a protein produced as a result of an abnormal gene fusion. Bosutinib targets BCR-ABL and is approved to treat CML that harbors the fusion protein. To be eligible for bosutinib, the cancer must also be in the chronic phase (an early stage of CML) and must be newly diagnosed or resistant or intolerant to prior CML therapies.
Some molecular alterations may not serve as drug targets but can indicate sensitivity to a targeted therapy, thereby serving as biomarkers of a potential response. This quarter, the FDA approved new indications for two drugs based on such biomarkers.
- In July, the FDA approved dostarlimab-gxly (Jemperli) for patients with advanced or recurrent endometrial cancer whose tumors have DNA mismatch repair deficiencies (a defect in DNA damage repair) or high microsatellite instability (a marker of a relatively unstable genome). Both characteristics are associated with improved response to immune checkpoint inhibitors such as dostarlimab, which targets the immune checkpoint protein PD-1.
Dostarlimab was approved in February 2023 to treat advanced or recurrent endometrial cancer that did not respond to or relapsed following treatment with platinum chemotherapy. The current approval allows for combination therapy containing dostarlimab, carboplatin, and paclitaxel to be used in the first-line setting, followed by maintenance therapy with dostarlimab alone.
- In August, the FDA approved a combination therapy (Akeega) consisting of niraparib and abiraterone acetate for the treatment of metastatic castration-resistant prostate cancer harboring mutations in the genes BRCA1 or BRCA2. The BRCA genes are part of the homologous recombination pathway, a critical DNA repair pathway. When the BRCA genes are mutated, the homologous recombination pathway does not function properly, leaving tumor cells reliant on another type of DNA repair called nonhomologous end joining (NHEJ).
Niraparib (Zejula) is a PARP inhibitor, which blocks the NHEJ pathway, causing catastrophic DNA damage in BRCA-mutated cells. Abiraterone is a type of hormone therapy that blocks the production of testosterone, which can drive prostate cancer growth. The approved treatment regimen also contains prednisone, a steroid that can mitigate some side effects of this therapy.
New Immunotherapies for Multiple Myeloma
Antibodies like dostarlimab work by keeping molecules, like a tumor-promoting protein and its receptor, apart. Other antibodies work by bringing molecules together.
Bispecific T-cell engagers (BiTEs) are composed of two antibodies—one that binds to proteins on a cancer cell and one that binds to proteins on a T cell. The BiTE helps the T cell kill the cancer cell by bringing the two together.
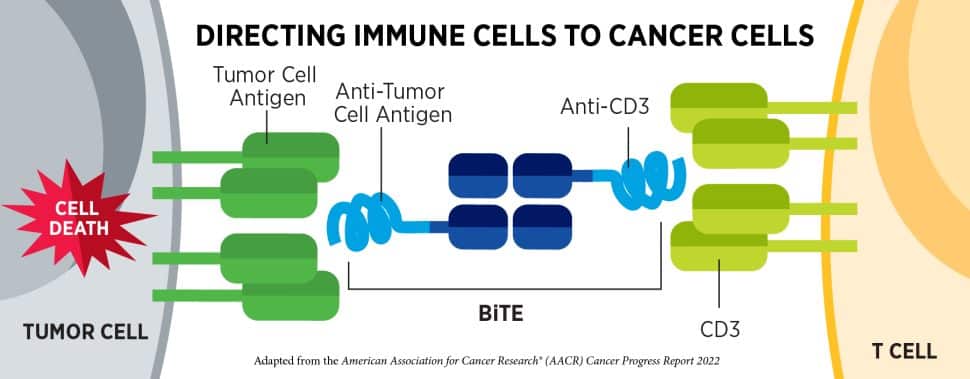
Last quarter, the FDA granted accelerated approval to two BiTEs for patients with certain relapsed or refractory B-cell lymphomas. This quarter, they granted accelerated approval to two BiTEs for patients with multiple myeloma, a cancer that arises from immune cells called plasma cells.
A formidable arsenal of therapies has been approved to fight multiple myeloma, including chemotherapy, stem cell transplants, immunomodulatory drugs (which elicit nonspecific effects on the immune system), histone deacetylase inhibitors (which disrupt gene expression), proteasome inhibitors (which prevent the cell from breaking down proteins it no longer needs), and two types of CAR T-cell therapy. Nevertheless, some multiple myelomas relapse after these therapies or don’t respond to them.
BiTEs entered the multiple myeloma space when teclistimab (Tecvayli) received an accelerated approval in 2022 to treat multiple myeloma that did not respond to or recurred following four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory drug, and an antibody targeting CD38. Teclistimab targets the B-cell maturation antigen (BCMA) on myeloma cells and CD3 on T cells.
The BiTEs approved this quarter are also intended for tumors that were previously treated with four prior lines of therapy, but they differ in the ways they bind their target cells.
- Elranatamab-bcmm (Elrexfio) was granted accelerated approval in August and, like teclistamab, it targets CD3 and BCMA. At the AACR Annual Meeting 2023, held April 14-19, researchers explained that elranatamab does not bind as tightly to CD3 as other BiTEs, which may decrease T-cell exhaustion and the incidence of a potentially life-threatening side effect called cytokine release syndrome.
- Talquetamab-tgvs (Talvey) was also granted accelerated approval in August, and while it, too, targets CD3, its target on multiple myeloma cells is the receptor GPRC5D. Talquetamab marks the first approval of a GPRC5D-targeted therapy, although other BiTEs and a handful of CAR T cells targeting GPRC5D are in clinical development.
Creative Approaches to Chemotherapy
Chemotherapy has been a mainstay of cancer treatment since 1949, when mechlorethamine (Mustargen), a key component of mustard gas, became the first chemical agent approved by the FDA to fight cancer.
In the time since, a vast array of chemotherapeutics has emerged with a variety of mechanisms, from the disruption of DNA replication to the destabilization of the cell’s internal skeleton. Because chemotherapies typically target rapidly dividing cells, they risk damaging certain types of healthy cells as well.
In recent years, researchers have improved chemotherapy efficacy in some tumor types by combining it with targeted therapies, and they have improved chemotherapy specificity by developing new drug delivery mechanisms. The FDA approved two novel approaches to chemotherapy this quarter and updated the indications of temozolomide (Temovar) via FDA’s Project Renewal program.
- In August, the FDA approved the chemotherapy combination trifluridine and tipiracil (Lonsurf), with the targeted therapy bevacizumab (Avastin), for the treatment of metastatic colorectal cancer following several rounds of prior chemotherapy.
Trifluridine inhibits DNA polymerase, the enzyme responsible for DNA replication. If cancer cells cannot replicate their DNA, they cannot divide, and they die. Tipiracil boosts the efficacy of trifluridine. Studies have also suggested that tipiracil might decrease cell migration and angiogenesis, the formation of new blood vessels around the tumor.
The combination of trifluridine and tipiracil was previously approved for metastatic, heavily pretreated colorectal cancer in 2015 and approved for gastric or gastroesophageal junction cancer in 2019. The current approval is the first to combine trifluridine and tipiracil with bevacizumab, an inhibitor of the vascular endothelial growth factor (VEGF), which can promote tumor growth and stimulate angiogenesis.
- Later in August, the FDA approved a kit (Hepzato Kit) to deliver the chemotherapy melphalan directly to the liver for the treatment of uveal melanoma metastases. While melphalan has been approved to treat cancer since 1964, this is the first formulation of melphalan designed specifically for hepatic delivery.
Melphalan cross-links strands of DNA together, preventing their replication and transcription, which can result in the death of rapidly dividing cells. Like many chemotherapeutics, systemic administration of melphalan can cause side effects such as gastrointestinal upset, hair loss, mouth sores, and immune suppression. Since liver cells do not divide rapidly, the delivery of melphalan directly to the liver minimizes side effects and enables the use of a higher dose.
While uveal melanoma is a type of skin cancer that originates in the eye, metastasis, especially to the liver, is common. Intrahepatic melphalan is intended for patients with little to no disease outside the liver and for whom 50% of the liver does not have metastases. The kit contains a device that allows for infusion of melphalan directly into the hepatic artery, limiting extrahepatic circulation.
- In September, the FDA approved new and updated indications of temozolomide as part of an Oncology Center of Excellence initiative called Project Renewal. While most FDA approvals happen during the marketing period for a new drug, off-patent drugs that have existed for decades are no longer evaluated in large clinical trials. However, smaller studies investigating off-label uses for these drugs can inform new approvals. Project Renewal seeks to analyze real-world data to update the approved indications for older drugs. AACR is a strategic partner of Project Renewal, providing scientific advice and perspective.
This approval includes a new indication for temozolomide for the treatment of newly diagnosed anaplastic astrocytoma, a type of brain tumor, and revised indications for the treatment of relapsed or refractory anaplastic astrocytoma. Temozolomide is also approved to treat newly diagnosed glioblastoma.
For more details, including the full indications and the clinical trial results that led to each approval, visit the FDA approvals page on the AACR website.



