Editors’ Picks: October Highlights From the AACR Journals
As you prepare for trick-or-treating, check out the articles selected by the editors of the 10 AACR journals for the month of October. Highlights include studies on the regulation of electrical activity between melanoma cells and keratinocytes; the role of cancer care providers in HPV vaccine delivery among pediatric, adolescent, and young adult cancer survivors; how fatty acids support the fitness of tumor-infiltrating T cells; and two clinical trials in liver cancer.
The abstracts of these studies are included below, and the full articles will be freely available for a limited time.
Journal: Blood Cancer Discovery
Cancer initiation is orchestrated by an interplay between tumor-initiating cells and their stromal/immune environment. Here, by adapted single-cell RNA sequencing, we decipher the predicted signaling between tissue-resident hematopoietic stem/progenitor cells (HSPC) and their neoplastic counterparts with their native niches in the human bone marrow. LEPR+ stromal cells are identified as central regulators of hematopoiesis through predicted interactions with all cells in the marrow. Inflammatory niche remodeling and the resulting deprivation of critical HSPC regulatory factors are predicted to repress high-output hematopoietic stem cell subsets in NPM1-mutated acute myeloid leukemia (AML), with relative resistance of clonal cells. Stromal gene signatures reflective of niche remodeling are associated with reduced relapse rates and favorable outcomes after chemotherapy across all genetic risk categories. Elucidation of the intercellular signaling defining human AML, thus, predicts that inflammatory remodeling of stem cell niches drives tissue repression and clonal selection but may pose a vulnerability for relapse-initiating cells in the context of chemotherapeutic treatment.
Significance: Tumor-promoting inflammation is considered an enabling characteristic of tumorigenesis, but mechanisms remain incompletely understood. By deciphering the predicted signaling between tissue-resident stem cells and their neoplastic counterparts with their environment, we identify inflammatory remodeling of stromal niches as a determinant of normal tissue repression and clinical outcomes in human AML.
This study was highlighted and featured on the cover of the October issue. A related commentary is available here.
Journal: Cancer Discovery
GABA Regulates Electrical Activity and Tumor Initiation in Melanoma
Oncogenes can initiate tumors only in certain cellular contexts, which is referred to as oncogenic competence. In melanoma, whether cells in the microenvironment can endow such competence remains unclear. Using a combination of zebrafish transgenesis coupled with human tissues, we demonstrate that GABAergic signaling between keratinocytes and melanocytes promotes melanoma initiation by BRAFV600E.
GABA is synthesized in melanoma cells, which then acts on GABA-A receptors in keratinocytes. Electron microscopy demonstrates specialized cell-cell junctions between keratinocytes and melanoma cells, and multielectrode array analysis shows that GABA acts to inhibit electrical activity in melanoma/keratinocyte cocultures. Genetic and pharmacologic perturbation of GABA synthesis abrogates melanoma initiation in vivo. These data suggest that GABAergic signaling across the skin microenvironment regulates the ability of oncogenes to initiate melanoma.
Significance: This study shows evidence of GABA-mediated regulation of electrical activity between melanoma cells and keratinocytes, providing a new mechanism by which the microenvironment promotes tumor initiation. This provides insights into the role of the skin microenvironment in early melanomas while identifying GABA as a potential therapeutic target in melanoma.
This study was highlighted and featured on the cover of the October issue. A related commentary is available here. For additional perspectives from the authors, check out this blog post.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Background: Geographic variability in esophageal cancer has been reported in China, but data are lacking at the local level. We aimed to investigate changes in disparities in esophageal cancer-related mortality among Chinese counties and whether county-level socioeconomic status was associated with this variation.
Methods: We used data from a nationwide survey and population-based cancer registries to calculate esophageal cancer-related mortality rates for 782 Chinese counties for the periods of 1973-1975 and 2015-2017. We performed hotspot analysis to identify spatial clusters. We used a multivariable negative binomial regression model to estimate the associations between county-level socioeconomic factors and mortality.
Results: From 1973-1975 to 2015-2017, the age-standardized esophageal cancer-related mortality rate decreased from 27 to 8 per 100,000 person-years in China. By county, 577 (74%) of 782 counties experienced decreasing mortality. Geographic disparities in mortality substantially narrowed, with the gap in mortality rates between 90th and 10th percentile counties decreasing from 55 per 100,000 person-years in 1973-1975 to 16 in 2015-2017. However, clusters of elevated rates persisted across north-central China. Rurality [adjusted mortality rate ratio (MRR) 1.15; 95% confidence interval (CI), 1.10–1.21], per capita gross domestic product (adjusted MRR, 0.95; 95% CI, 0.91–0.98), and percentage of people with a high-school diploma (adjusted MRR, 0.86; 95% CI, 0.84–0.87) in a county were significantly associated esophageal cancer-related mortality rates.
Conclusions: China has made substantial progress in reducing esophageal cancer-related mortality and disparities, but the intercounty differences remain large.
Impact: Continued efforts are needed to address the geographical and socioeconomic disparities in esophageal cancer.
This study was highlighted in the October issue.
Journal: Cancer Immunology Research
Glioblastoma (GBM) is the deadliest form of brain cancer. It is a highly angiogenic and immunosuppressive malignancy. Although immune checkpoint blockade therapies have revolutionized treatment for many types of cancer, their therapeutic efficacy in GBM has been far less than expected or even ineffective. In this study, we found that the genomic signature of glioma-derived endothelial cells (GdEC) correlates with an immunosuppressive state and poor prognosis of patients with glioma. We established an in vitro model of GdEC differentiation for drug screening and used this to determine that cyclic adenosine monophosphate (cAMP) activators could effectively block GdEC formation by inducing oxidative stress. Furthermore, cAMP activators impaired GdEC differentiation in vivo, normalized the tumor vessels, and altered the tumor immune profile, especially increasing the influx and function of CD8+ effector T cells. Dual blockade of GdECs and PD-1 induced tumor regression and established antitumor immune memory. Thus, our study reveals that endothelial transdifferentiation of GBM shapes an endothelial immune cell barrier and supports the clinical development of combining GdEC blockade and immunotherapy for GBM.
A commentary related to this study is available here.
Journal: Cancer Prevention Research
Although pediatric, adolescent, and young adult (PAYA) cancer survivors are at increased risks for secondary cancers, their HPV vaccine uptake rates are poor. Therefore, we conducted a mixed-methods study to identify the barriers and opportunities for HPV vaccine delivery among PAYA cancer care providers. We distributed a semistructured questionnaire to a professional organization comprised of PAYA oncology and hematology healthcare providers between April and July 2022. Questionnaire measures included demographic and practice characteristics, HPV vaccine knowledge, willingness, barriers, opportunities, and roles for HPV vaccine delivery. Descriptive characteristics were generated for quantitative data, and content analysis was used to identify themes. A total of 49 providers responded to our survey. A majority were female (68%) and non-Hispanic white (74%). Approximately 76% were oncology or hematology physicians, and most worked in a cancer center or children’s hospital (86%). Over half (63%) had been practicing for >15 years, and a majority saw patients ages 11 to 17. Although less than half reported discussing HPV vaccination with their patients, 69% were willing to become involved in HPV vaccine delivery. The most frequently reported barriers identified in our content analysis were related to system-level factors. Furthermore, providers identified opportunities within cancer prevention education, transitions in care, and at the system-level. Although barriers to HPV vaccination persist in cancer care, most providers perceived there to be opportunities to become involved in HPV vaccine delivery. Identifying strategies for PAYA oncology and hematology healthcare providers to adopt a stronger role in HPV vaccination remains a significant opportunity for future implementation research.
Prevention Relevance: This mixed-methods study is the first to investigate and assess barriers and opportunities for HPV vaccine delivery among PAYA cancer healthcare providers. Our findings can serve as an important framework for future implementation research targeted towards HPV vaccine delivery in cancer clinical settings.
A commentary related to this study is available here.
Journal: Cancer Research (October 1 issue)
Kinome Reprogramming Is a Targetable Vulnerability in ESR1 Fusion-driven Breast Cancer
Transcriptionally active ESR1 fusions (ESR1-TAF) are a potent cause of breast cancer endocrine therapy (ET) resistance. ESR1-TAFs are not directly druggable because the C-terminal estrogen/anti-estrogen-binding domain is replaced with translocated in-frame partner gene sequences that confer constitutive transactivation. To discover alternative treatments, a mass spectrometry (MS)-based kinase inhibitor pulldown assay (KIPA) was deployed to identify druggable kinases that are upregulated by diverse ESR1-TAFs. Subsequent explorations of drug sensitivity validated RET kinase as a common therapeutic vulnerability despite remarkable ESR1-TAF C-terminal sequence and structural diversity. Organoids and xenografts from a pan-ET-resistant patient-derived xenograft model that harbors the ESR1-e6>YAP1 TAF were concordantly inhibited by the selective RET inhibitor pralsetinib to a similar extent as the CDK4/6 inhibitor palbociclib. Together, these findings provide preclinical rationale for clinical evaluation of RET inhibition for the treatment of ESR1-TAF-driven ET-resistant breast cancer.
Significance: Kinome analysis of ESR1 translocated and mutated breast tumors using drug bead-based mass spectrometry followed by drug-sensitivity studies nominates RET as a therapeutic target.
A commentary related to this study is available here.
Journal: Cancer Research (October 15 issue)
CD8+ tissue-resident memory T (Trm) cells and tumor-infiltrating lymphocytes (TIL) regulate tumor immunity and immune surveillance. Characterization of Trm cells and TILs could help identify potential strategies to boost antitumor immunity. Here, we found that the transcription factor SCML4 was required for the progression and polyfunctionality of Trm cells and was associated with a better prognosis in patients with cancer. Moreover, SCML4 maintained multiple functions of TILs. Increased expression of SCML4 in CD8+ cells significantly reduced the growth of multiple types of tumors in mice, while deletion of SCML4 reduced antitumor immunity and promoted CD8+ T-cell exhaustion. Mechanistically, SCML4 recruited the HBO1-BRPF2-ING4 complex to reprogram the expression of T cell-specific genes, thereby enhancing the survival and effector functions of Trm cells and TILs. SCML4 expression was promoted by fatty acid metabolism through mTOR-IRF4-PRDM1 signaling, and fatty acid metabolism-induced epigenetic modifications that promoted tissue-resident and multifunctional gene expression in Trm cells and TILs. SCML4 increased the therapeutic effect of anti-PD-1 treatment by elevating the expression of effector molecules in TILs and inhibiting the apoptosis of TILs, which could be further enhanced by adding an inhibitor of H3K14ac deacetylation. These results provide a mechanistic perspective of functional regulation of tumor-localized Trm cells and TILs and identify an important activation target for tumor immunotherapy.
Significance: SCML4 upregulation in CD8+ Trm cells and tumor-infiltrating lymphocytes induced by fatty acid metabolism enhances antitumor immune responses, providing an immunometabolic axis to target for cancer treatment.
A commentary related to this study is available here.
Journal: Clinical Cancer Research (October 1 issue)
A Phase II Study of Optimized Individualized Adaptive Radiotherapy for Hepatocellular Carcinoma
Purpose: We hypothesized that optimizing the utility of stereotactic body radiotherapy (SBRT) based on the individual patient’s probability for tumor control and risk of liver injury would decrease toxicity without sacrificing local control in patients with impaired liver function or tumors not amenable to thermal ablation.
Patients and Methods: Patients with Child-Pugh (CP) A to B7 liver function with aggregate tumor size >3.5 cm, or CP ≥ B8 with any size tumor were prospectively enrolled on an Institutional Review Board-approved phase II clinical trial to undergo SBRT with baseline and midtreatment dose optimization using a quantitative, individualized utility-based analysis. Primary endpoints were change in CP score of ≥2 points within 6 months and local control. Protocol-treated patients were compared with patients receiving conventional SBRT at another cancer center using overlap weighting.
Results: A total of 56 patients with 80 treated tumors were analyzed with a median follow-up of 11.2 months. Two-year cumulative incidence of local progression was 6.4% [95% confidence interval (CI, 2.4–13.4)]. Twenty-one percent of patients experienced treatment-related toxicity within 6 months, which is similar to the rate for SBRT in patients with CP A liver function. An analysis using overlap weighting revealed similar local control [HR, 0.69; 95% CI (0.25–1.91); P = 0.48] and decreased toxicity [OR, 0.26; 95% CI (0.07–0.99); P = 0.048] compared with conventional SBRT.
Conclusions: Treatment of individuals with impaired liver function or tumors not amenable to thermal ablation with a treatment paradigm designed to optimize utility may decrease treatment-related toxicity while maintaining tumor control.
This study was highlighted in the October 1 issue.
Journal: Clinical Cancer Research (October 15 issue)
Purpose: This study aimed to evaluate the efficacy and safety of camrelizumab plus apatinib with or without stereotactic body radiotherapy (SBRT) as first-line therapy for patients with hepatocellular carcinoma (HCC) with portal vein tumor thrombus (PVTT).
Patients and Methods: This is a multicenter, open-label, noncomparative, randomized trial that recruited patients with HCC with type II/III/IV PVTT, who had not previously received systemic therapy. Patients were randomly assigned (2:1) to receive camrelizumab (200 mg, every 3 weeks) and apatinib (250 mg, every day) with or without SBRT [95% planning target volume (PTV), 36-40 Gy/6-8 Gy]. The primary endpoint was overall survival (OS), and the secondary endpoints were progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), duration of response, time to progression, and safety.
Results: Sixty patients were enrolled and randomly assigned to two prospective cohorts. Median OS were 12.7 months [95% confidence interval (CI), 10.2-not available (NA)] and 8.6 months (95% CI, 5.6–NA), and median PFS were 4.6 months (95% CI, 3.3–7.0) and 2.5 months (95% CI, 2.0-7.6) for the SBRT and non-SBRT cohorts, respectively. The ORR and DCR were 47.5% and 72.5% in the SBRT cohort, and 20.0% and 40.0% in the non-SBRT cohort. The most common treatment-related adverse events of any grade were hypertension (55.0%), hand-foot syndrome (51.7%), and leukopenia (50.0%). Grade ≥ 3 was reported in 13 (21.7%) patients.
Conclusions: First-line treatment with camrelizumab-apatinib combined with or without SBRT showed clinical benefits in patients with HCC with PVTT, with an acceptable safety profile. Thus, these combination regimens may be potential options for such patients.
This study was highlighted in the October 15 issue.
Journal: Molecular Cancer Research
Activating estrogen receptor alpha (ER; also known as ESR1) mutations are present in primary endometrial and metastatic breast cancers, promoting estrogen-independent activation of the receptor. Functional characterizations in breast cancer have established unique molecular and phenotypic consequences of the receptor, yet the impact of ER mutations in endometrial cancer has not been fully explored. In this study, we used CRISPR-Cas9 to model the clinically prevalent ER-Y537S mutation and compared results with ER-D538G to discover allele-specific differences between ER mutations in endometrial cancer. We found that constitutive activity of mutant ER resulted in changes in the expression of thousands of genes, stemming from combined alterations to ER binding and chromatin accessibility. The unique gene expression programs resulted in ER-mutant cells developing increased cancer-associated phenotypes, including migration, invasion, anchorage-independent growth, and growth in vivo. To uncover potential treatment strategies, we identified ER-associated proteins via Rapid Immunoprecipitation and Mass Spectrometry of Endogenous Proteins and interrogated two candidates, CDK9 and NCOA3. Inhibition of these regulatory proteins resulted in decreased growth and migration, representing potential novel treatment strategies for ER-mutant endometrial cancer.
Implications: This study provides insight into mutant ER activity in endometrial cancer and identifies potential therapies for women with ER-mutant endometrial cancer.
This study was highlighted in the October issue.
Journal: Molecular Cancer Therapeutics
Dual Targeting of the PDZ1 and PDZ2 Domains of MDA-9/Syntenin Inhibits Melanoma Metastasis
Genome-wide gene expression analysis and animal modeling indicate that melanoma differentiation associated gene-9 (mda-9, Syntenin, Syndecan binding protein, referred to as MDA-9/Syntenin) positively regulates melanoma metastasis. The MDA-9/Syntenin protein contains two tandem PDZ domains serving as a nexus for interactions with multiple proteins that initiate transcription of metastasis-associated genes. Although targeting either PDZ domain abrogates signaling and prometastatic phenotypes, the integrity of both domains is critical for full biological function. Fragment-based drug discovery and NMR identified PDZ1i, an inhibitor of the PDZ1 domain that effectively blocks cancer invasion in vitro and in vivo in multiple experimental animal models.
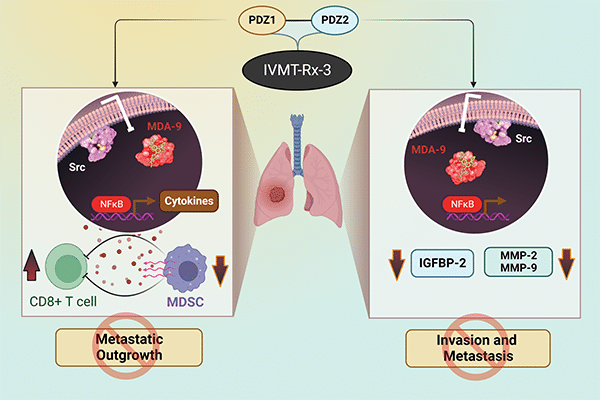
To maximize disruption of MDA-9/Syntenin signaling, an inhibitor has now been developed that simultaneously binds and blocks activity of both PDZ domains. PDZ1i was joined to the second PDZ binding peptide (TNYYFV) with a PEG linker, resulting in PDZ1i/2i (IVMT-Rx-3) that engages both PDZ domains of MDA-9/Syntenin. IVMT-Rx-3 blocks MDA-9/Syntenin interaction with Src, reduces NF-κB activation, and inhibits MMP-2/MMP-9 expression, culminating in repression of melanoma metastasis. The in vivo antimetastatic properties of IVMT-Rx-3 are enhanced when combined with an immune-checkpoint inhibitor. Collectively, our results support the feasibility of engineering MDA-9 dual-PDZ inhibitors with enhanced antimetastatic activities and applications of IVMT-Rx-3 for developing novel therapeutic strategies effectively targeting melanoma and in principle, a broad spectrum of human cancers that also overexpress MDA-9/Syntenin.
This study was highlighted and featured on the cover of the October issue.
Journal: Cancer Research Communications
The FOXA1 pioneer factor is an essential mediator of steroid receptor function in multiple hormone-dependent cancers, including breast and prostate cancers, enabling nuclear receptors such as estrogen receptor (ER) and androgen receptor (AR) to activate lineage-specific growth programs. FOXA1 is also highly expressed in non-small cell lung cancer (NSCLC), but whether and how it regulates tumor growth in this context is not known. Analyzing data from loss-of-function screens, we identified a subset of NSCLC tumor lines where proliferation is FOXA1 dependent. Using rapid immunoprecipitation and mass spectrometry of endogenous protein, we identified chromatin-localized interactions between FOXA1 and glucocorticoid receptor (GR) in these tumor cells. Knockdown of GR inhibited proliferation of FOXA1-dependent, but not FOXA1-independent NSCLC cells. In these FOXA1-dependent models, FOXA1 and GR cooperate to regulate gene targets involved in EGF signaling and G1-S cell-cycle progression. To investigate the therapeutic potential for targeting this complex, we examined the effects of highly selective inhibitors of the GR ligand-binding pocket and found that GR antagonism with ORIC-101 suppressed FOXA1/GR target expression, activation of EGF signaling, entry into the S-phase, and attendant proliferation in vitro and in vivo. Taken together, our findings point to a subset of NSCLCs harboring a dependence on the FOXA1/GR growth program and provide rationale for its therapeutic targeting.
Significance: NSCLC is the leading cause of cancer deaths worldwide. There is a need to identify novel druggable dependencies. We identify a subset of NSCLCs dependent on FOXA1-GR and sensitive to GR antagonism.