Experts Forecast 2024, Part 4: Cutting-edge Tech for Oncology Drug Discovery
In anticipation of the new year, we asked four experts to predict the advances we might see in 2024 in their respective fields. In part 4 of this four-part series, we share thoughts from Paul Workman, PhD, on advances in oncology drug discovery.
Check out the previous installments of the series:
- Part 1 featured predictions from Catherine J. Wu, MD, FAACR, for cancer vaccines
- Part 2 featured predictions from Robert A. Winn, MD, FAACR, for achieving health equity
- Part 3 featured predictions from AACR President-Elect Patricia M. LoRusso, DO, PhD (hc), FAACR, for precision medicine
New therapeutics can be a driving force in extending patient survival and reducing deaths from cancer. Last year alone, the U.S. Food and Drug Administration (FDA) approved 17 new cancer drugs and expanded prior approvals for several others.
Despite this progress, drug discovery still has a long way to go, said Paul Workman, PhD, a professor and drug discovery expert at The Institute of Cancer Research in London.
“Ninety-five percent of cancer drugs tested in clinical trials never get approved,” he said. “This is because they end up being too toxic, ineffective, or no better than existing therapies once they are evaluated in patients, despite promising preclinical data.”

Moreover, only 14% of cancer patients in the United States are treated with precision medicine, and only 7% benefit—a sobering reality that Workman attributes in large part to the limited number of cancer drivers that are currently druggable. “The main reason for the low application of precision medicine is that most cancers are driven by genetic alterations for which we currently have no drugs.
“So, number one, we need to increase the clinical success rate of the drugs we are developing, and number two, we need to find ways to discover drugs against the so-called ‘undruggable’ targets,” he said. “In addition, we need to use intelligent combinations of new and old drugs to overcome drug resistance.”
Before new small-molecule drugs can reach the clinic, they must be designed to selectively block the function of the desired target protein and undergo extensive testing in the lab. This relies on researchers being able to identify new targets and engineer compounds against them that are safe and effective.
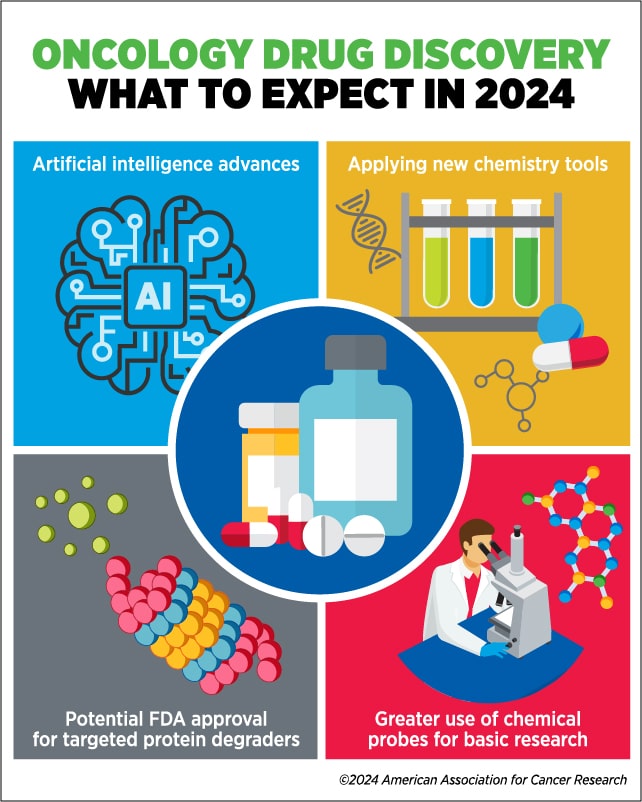
“This is where new technologies will help us,” Workman said. In 2024, he predicts that rapid advances in artificial intelligence (AI), chemical technology, and small-molecule chemical probes or tools will provide the cutting-edge resources needed to expand and accelerate drug discovery for cancer.
Artificial Intelligence
The November 2022 release of ChatGPT made AI a household topic last year. But, in drug discovery, a huge advance of AI came earlier—in 2021, researchers unveiled AlphaFold, an AI algorithm that can accurately predict the structures of more than 90% of proteins.
Understanding a target protein’s precise atomic structure is key for researchers when attempting to develop small-molecule drugs against it. Ideally, they should know the presence and location of potentially druggable pockets and grooves into which a drug could fit. Traditional methods of determining protein structure can take years and, for many proteins, may even be unsuccessful.
AlphaFold applies decades of data from previously solved protein structures deposited in the public Protein Data Bank to predict the structures of unsolved proteins. In the future, Workman anticipates that AI algorithms like AlphaFold may even be able to routinely solve the structures of protein complexes, allowing researchers to better target cellular processes and predict the folding of ligands (precursors of drugs) in the presence of target proteins. Workman says that this research is already underway, with initial signs of success and huge potential.
“AlphaFold could shave years off the initial phase of the oncology drug discovery pathway,” said Workman, noting that it will enable researchers to design drugs for new targets without having to wait for a crystal structure to be solved. “In some cases, it may allow researchers to design drugs against targets that couldn’t have been worked on at all before—which will have even bigger impact.
“AlphaFold will continue to be transformative for predicting protein structure and finding binding ligands, but let’s not get too overexcited—currently, it only really helps with the very early stages of the drug discovery pathway.”
AI, however, is already contributing to downstream steps in the drug discovery process, Workman said. “The first drugs are already entering the clinic for which AI has greatly shortened length and reduced the cost of the medicinal chemistry optimization phase.
“I predict that, in the near future, there will be an AI algorithm that can not only predict the structure of a protein but also design small molecules that bind to it,” he speculated.
“And once we have a test compound, wouldn’t it be great if AI could predict its pharmacokinetics and safety profile in humans as well?” Developing such an algorithm, however, would require training it on pharmacokinetic and toxicity data of existing compounds—data that are mostly guarded by pharmaceutical companies and not widely shared.
“If pharmaceutical companies and academic drug discoverers can put all of their pharmacokinetic, pharmacodynamic, and toxicology data into the public domain, it would revolutionize AI’s capacity to predict the clinical efficacy and safety of drugs. This is how we can increase the success rate of clinical testing,” he said. “Ideally, AI would also be applied to shorten and enhance the expensive rate-limiting late-stage clinical trials beyond its already demonstrated impact on digital pathology and imaging.”
Finally, Workman is excited about the prospect of digital twins, which he describes as highly sophisticated in silico models of individual patients.
“As an example, you could build the digital twin of a patient and then test how a particular drug might impact that patient’s cancer before prescribing it to them,” he explained. “I think the time will come when we will be doing much more experimentation in silico and much less in vivo, making drug discovery cheaper, faster, and more successful.”
Discoveries Fueled by Chemistry Advances
Workman also predicts that small-molecule drug discovery will benefit from recent leaps in powerful new technologies, such as advances in solving the structures of protein complexes.
Because proteins function through interactions with other proteins, he noted that determining how proteins are situated when in complex with one another can be critical to developing effective drugs.
“Researchers can now determine the structures of very large complexes using cryo-electron microscopy (cryoEM) and study how an investigational drug fits into a given protein complex,” Workman explained. “This provides them with information that can be used to enable and enhance drug binding.”
Designing and optimizing drugs will benefit from revolutionary advances in chemical technology as well. DNA-encoded chemical libraries, for example, contain a large collection of diverse chemical compounds that can be tested for their ability to bind to a target of interest. Each compound is tagged with a DNA barcode for easy identification.
“Compared to conventional screening methods, which might test tens or hundreds of thousands of compounds, DNA-encoded chemical libraries allow researchers to test millions or more of chemical compounds at once—very helpful for tackling tougher drug targets,” said Workman.
Workman also identified fragment-based drug design—where a drug is engineered by starting with small chemical fragments and then joining or expanding these—as another approach that is already helping to streamline drug discovery, especially when coupled to crystallography.
“Instead of starting with a collection of bigger compounds, you can take these tiny fragments that fit inside any active site and then build up the drug structure bit by bit to achieve an optimal fit,” he explained. The advantage of this method is that tiny fragments are easier to fit into smaller pockets and grooves as compared to the larger molecules that are commonly present in conventional screening libraries.
He also expects that chemical compounds known as macrocycles, which contain large rings in their structure, will facilitate drug design due to their ability to fit into hard-to-drug pockets and to enable development of drugs beyond the traditional “Lipinski’s rule of 5” parameters.
Finally, Workman pointed to covalently binding inhibitors and targeted protein degraders—such as proteolysis targeting chimeras (PROTACs) and molecular glues—as recent examples of chemical technologies driving progress in drug development. Targeted protein degrader drugs induce the breakdown of cancer-driving proteins by binding the target and recruiting cellular degradation machinery.
“Being able to design protein degraders that effectively and selectively remove a protein of interest is probably the biggest chemistry breakthrough we’ve seen in oncology drug discovery in recent years,” he said, adding that these drugs have immense potential to tackle harder-to-drug cancer targets in 2024.
He noted that the success of targeted protein degraders lies in their transient binding to targets (which allows them to have catalytic activity and continually degrade their targets) and their ability to tolerate low binding affinities (which makes them more suitable than conventional reversibly acting inhibitor drugs).
“The key aspect is that you only need initial weak binding to the target to attract the degradation machinery, which then degrades the protein very effectively even with weak binding,” he explained.
While some protein degraders—thalidomide (Thalomid) and its derivatives—are already approved for multiple myeloma, these drugs were not initially designed as protein degraders (their mechanism of action was uncovered decades after development). Currently, no PROTACs or molecular glues designed against a specific target are approved for clinical use.
“This year, I expect that we will have the first FDA approval for a ‘designer’ targeted protein degrader, and in the coming five years, I expect that more and more of these drugs will enter the clinic.”
Chemical Probes in Basic Research for Drug Discovery
Oncology drug development relies on understanding basic cellular processes and how they are disrupted in cancer. Scientists often use small-molecule tools, usually inhibitors, in basic research to determine the function of cellular proteins. But in 2024, Workman anticipates even wider use of such chemical probes to address basic biological questions, validate drug targets, help assess druggability, and act as pathfinders for novel drug classes.
“In contrast to clinical drugs which can often hit multiple protein targets, chemical probes can be readily used in the lab as long as they bind very potently and selectively to their targets and are cell permeable; there is no need to sort out oral absorption rates, systemic pharmacokinetics or in vivo tolerability—unless the tool is to be used in, say, mice,” he said.
Chemical probes could also be a faster and more efficient orthogonal alternative to genetic knockouts or CRISPR-based gene editing, Workman added. “The beauty of a small-molecule tool is that, so long as it’s well designed, highly selective, well characterized, and cell penetrant, a researcher can simply put it onto cells and immediately determine the impact of blocking a target protein before taking on the expense, effort, and risk of designing a drug against it—which can then follow later if the results look promising.
“In 2024, we urgently need to increase the number of high-quality chemical probes available to basic researchers and drug discoverers, especially achieving greater proteome coverage,” he said. To this end, he and colleagues launched the Chemical Probes Portal, a public database intended to help researchers find appropriate probes for their experiments; they are also developing criteria and guidelines for best-practice use of chemical probes.
“Underpinning all the breakthroughs to come are the myriad resources and technologies that scientists and clinicians are now equipped with—which allow them to answer fundamental biological questions about cancer, identify targets, and discover and develop drugs with much less experimentation than has traditionally been required,” Workman said.
“These advances will make drug discovery and hopefully also clinical drug development quicker and cheaper. They will allow us to be faster and much more successful than we’ve been in the past, especially with the tougher targets; this will ultimately benefit more patients than before—which is what it’s all about.”