TIL Therapy: A New Melanoma Treatment 30 Years in the Making
Almost two-thirds of patients diagnosed with metastatic melanoma are expected to die within five years, but a newly approved treatment may help some patients live longer. Lifileucel (Amtagvi), a tumor-infiltrating lymphocyte (TIL) therapy, is a first-of-its-kind personalized immunotherapy that amplifies the cancer-fighting immune cells already present in a patient’s melanoma.
Lifileucel is the first TIL therapy to be approved for clinical use and the first cell-based immunotherapy to be approved for a solid tumor.
Its historic approval is the culmination of more than 30 years of research, driven in large part by Steven A. Rosenberg, MD, PhD, FAACR, a senior investigator and chief of surgery at the National Cancer Institute, who received the 2024 AACR Award for Lifetime Achievement in Cancer Research for his pioneering contributions to the field of immunotherapy.
“Dr. Rosenberg’s innovative, groundbreaking research has revolutionized the scientific understanding of the immune response to cancer and resulted in the development of cancer immunotherapies and gene therapies that are saving countless lives around the world,” said Margaret Foti, PhD, MD (hc), chief executive officer of the AACR in a press release announcing the award.
Keep reading to learn about TIL therapy and the decades of basic, translational, and clinical research from Rosenberg and others that led to its momentous approval.
What is TIL Therapy?
Lifileucel and other TIL therapies under investigation work through the same basic principle: collect the patient’s tumor tissue through biopsy or surgery, isolate from the tissue the T cells that have infiltrated the tumor (called TILs), drastically increase the number of isolated TILs in the lab, and deliver the TILs back into the patient along with an infusion of the protein IL-2 to stimulate the TILs’ antitumor activity.
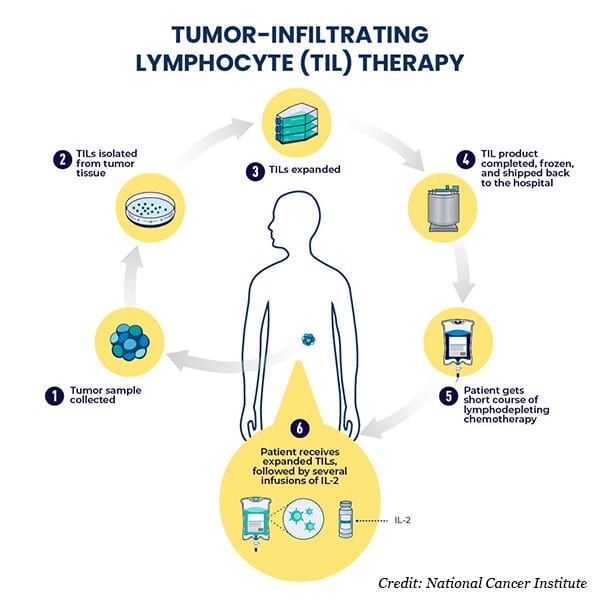
TIL therapy has several advantages over other immune cell-based therapies for treating solid tumors. For one, TILs have already found a way into the tumor, meaning they innately possess the features needed to bypass the difficult-to-permeate microenvironments of solid tumors. Second, since each patient’s TILs are already specific to their own tumor, the risk of off-target effects is low, and genetic modifications aren’t needed to endow tumor specificity to the cells. Moreover, the population of TILs isolated from a single patient typically includes multiple different TIL clones that can recognize various markers within the patient’s tumor. This may allow TILs to better respond to the heterogeneity of solid tumors compared with other immune cell-based therapies.
Discovery of TILs and Early Exploration
The story of TIL therapy can be traced back to 1986, when Rosenberg and colleagues reported the discovery of TILs in human tumors and a protocol to expand them in the lab using IL-2, which Rosenberg had previously identified to be a growth factor for T cells. When the human TILs were expanded and injected into mice, they led to regression of metastatic tumors in the liver and lungs.
These experiments also revealed that TILs were more potent and selective for tumors than a different type of immune cell (lymphokine-activated killer cells) that circulates in the blood and that had been previously explored for cancer therapy.
Two years later, Rosenberg and colleagues announced that 20 patients with metastatic melanoma had received infusions of their own TILs—the first time this experimental therapy had been used in humans. The TILs were administered alongside IL-2 with promising preliminary results: tumor regressions in 11 patients lasting from two to 13 months with toxicities that were reversible.
The final results from an expanded population of 86 patients were published in 1994, demonstrating a 34% response rate, but with only 7% of responses persisting one year after treatment.
Separate clinical trials that used radioactive or genetic markers to track the infused cells in patients showed that the TILs successfully homed to tumor sites within 24 hours but began declining in number after 21 days.
Overcoming Initial Challenges
While the studies of the 1980s and ’90s illustrated the promise of TILs for cancer therapy, they also revealed the shortcomings of this approach—namely the short-lived nature of treatment responses.
Despite the challenges, Rosenberg and others continued to explore the potential of TIL therapy. In the early 2000s, they found that using chemotherapy to deplete the patient’s own immune cells prior to TIL infusion (called lymphodepleting conditioning) increased response rates in a dose-dependent manner, possibly because the conditioning treatment also depleted cells that suppressed the immune response.
This adjustment led to a 56% response rate for patients with heavily treated metastatic melanoma, with over 20% of patients experiencing complete responses lasting at least three years—an improvement over prior trials in which only some patients had received lymphodepleting conditioning.

Rosenberg and colleagues also developed a simplified method for culturing TILs that helped keep the TILs active for longer. In an earlier clinical trial, they had observed that responses were more likely when patients were treated with TILs that had been in culture for less time. They reasoned that growing the TILs in the lab for an extended period was causing them to become “exhausted,” or inactive, soon after they were infused into the patient, thereby limiting their efficacy.
A new method reported in 2008—termed “Young TIL”—shortened the culture time and eliminated a step that screened for tumor-reactive TILs in the lab. The modifications prevented telomere shortening (a hallmark of T-cell exhaustion) and maintained high expression levels of the T-cell activation markers CD27 and CD28. The Young TIL protocol also overcame many of the technical challenges that could hinder the clinical utility of this treatment without adversely impacting response rates.
A New Treatment Landscape Forms
Over the following decade, multiple practice-changing therapies, including BRAF inhibitors and immune checkpoint inhibitors, were approved for melanoma, leading to a marked increase in survival rates. However, disease progression after these new therapies was not uncommon.
At the same time, TILs continued to show promise in various disease settings, including against cervical, breast, lung, and KRAS G12D-mutated colorectal cancers, and researchers were learning more about why some patients’ TILs were more effective than others. In 2020, Rosenberg and colleagues identified a particular subset of TILs—stem-like lymphocytes—responsible for antitumor activity.
By this point, it was becoming increasingly clear that TILs could treat melanomas that were unresponsive to established treatments, and in some cases, were even better than other treatment options. In 2022, TILs were found to be effective against metastatic melanomas refractory to prior treatment, with TIL therapy leading to superior outcomes in these patients compared with the immune checkpoint inhibitor ipilimumab (Yervoy).
By 2023, research was suggesting that TIL therapy could even lead to long-term survival benefits in patients with heavily pretreated melanomas.
It All Comes Together: FDA Approves First TIL Therapy
In December 2023, researchers reported that almost 50% of patients treated with an investigational TIL therapy called lifileucel were alive four years after treatment. The median duration of response had not been reached at the four-year follow-up, indicating the potential of long-term responses.
And on February 16, 2024, based on these data, the FDA granted accelerated approval to lifileucel for patients with unresectable or metastatic melanoma after prior treatment with a PD-1-targeted immune checkpoint inhibitor and, if eligible, a BRAF inhibitor. (Accelerated approval means that continued approval may be contingent upon a confirmatory trial.)
This monumental achievement was based on 30+ years of research, which begs the question:
What will the next 30 years bring for TILs?
Currently, researchers are exploring additional ways to improve TIL therapy and expand its clinical applications. Ongoing clinical trials are evaluating lifileucel for lung cancer and in combination with immune checkpoint inhibitors for the first-line treatment of melanoma.
Rosenberg and others are working to enhance tumor specificity by incorporating genetic modifications into investigational TILs, such as inserting stem-like markers or tumor antigen-specific T-cell receptors identified through new high-throughput sequencing technologies. Efforts are also underway to combine TIL therapy with vaccines, improve selection and growth conditions to identify stem-like TILs, enhance tumor reactivity by sensitizing TILs in culture, and identify more tumor antigens.
As Rosenberg delivered his award lecture at the AACR Annual Meeting 2024, he reflected on the progress he has witnessed throughout his career.
“In the 1970s when I first came to the National Cancer Institute,” he said, “there was no convincing evidence for human lymphocytes reactive with cancer, no knowledge of human cancer antigens, and certainly no successful immunotherapies for cancer in humans.”
Of course, since that time, dozens of immunotherapies have been approved to treat various types of cancer, many a result of work done by Rosenberg himself. Now, TILs have finally joined that list, and with recent advances in technology and the foundational work of the past few decades, researchers will hopefully be able to bring TIL therapy to even more patients, improving outcomes and, ultimately, saving lives.



