Tarlatamab-dlle Approved for Metastatic Lung Cancer
Tarlatamab-dlle is the first t-cell engager that uses the bispecific antibody design for a solid tumor.
The U.S. Food and Drug Administration (FDA) has granted accelerated approval to tarlatamab-dlle (Imdelltra) to treat patients with extensive-stage small cell lung cancer (ES-SCLC) that progressed during or after prior platinum-based chemotherapy.
Tarlatamab-dlle is a type of immunotherapy known as a bispecific T-cell engager (BiTE) that simultaneously binds proteins on both cancer cells and T cells (a type of immune cell). By doing so, it brings the two cell types together and allows the T cells to mount an immune response against the cancer cells. Tarlatamab-dlle recognizes the delta-like ligand 3 (DLL3) protein on cancer cells and the CD3 protein on T cells.
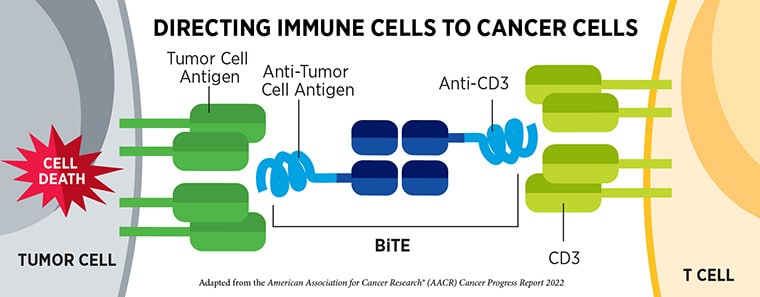
This approval—the first for tarlatamab-dlle—represents the first t-cell engager that uses the bispecific antibody design for a solid tumor.
The approval was based on results from DeLLphi-301, an open-label, multicenter, multicohort, phase II clinical trial in which 99 patients with relapsed or refractory ES-SCLC were treated with tarlatamab-dlle. All enrolled patients had previously experienced disease progression after platinum-based chemotherapy.
Forty percent of patients experienced an objective response to tarlatamab-dlle, with responses lasting for a median of 9.7 months.
The recommended dose is 1 mg on day 1, followed by 10 mg on days 8 and 15 and every two weeks after until disease progression or unacceptable toxicity. The drug should be administered intravenously over one hour.
The prescribing information for tarlatamab-dlle includes a boxed warning for cytokine release syndrome and neurological toxicities.
Small cell lung cancer is a fast-growing cancer that develops in the lungs. Extensive stage describes small cell lung cancers that have spread to other parts of the body. According to federal statistics, it was estimated that 234,580 individuals would be diagnosed with lung cancer and 125,070 patients would die of the disease in the United States in 2024.
The FDA rendered its decision on May 16, 2024. Accelerated approval means that continued approval may be contingent upon a confirmatory trial.