Improving Methods to Collect and Analyze Overall Survival Data
Since 1971, the number of cancer survivors living in the United States has more than tripled from 1.4% of the population (3 million survivors) to 5.4% of the population (18 million survivors). This progress emphasizes decades of success of basic and translational cancer research that have provided powerful new treatment options for patients. A new article published in Clinical Cancer Research highlights the increasing challenges that researchers face for quickly determining whether new drugs are safe and effective, with a focus on the gold standard endpoint overall survival (OS).
OS is considered the gold standard because how long a patient survives following a cancer therapy reflects both efficacy as well as safety, in addition to being readily understandable and relevant for patients. As patients with cancer live longer, OS data are becoming more difficult to collect and interpret in a timely fashion that promotes rapid drug development. Therefore, earlier endpoints such as progression-free survival (PFS) or tumor shrinkage metrics such as overall response rate (ORR) and minimum residual disease (MRD) negativity are becoming common measurements of treatment efficacy.
Unfortunately, early endpoints do not always correlate with OS. The U.S. Food and Drug Administration (FDA) has highlighted several examples where clinical trials had favorable early efficacy endpoints but detriments to OS. For example, the BELLINI trial tested the combination of venetoclax plus bortezomib vs. bortezomib alone for patients with multiple myeloma. Patients taking the combination therapy had outstanding early efficacy results with nearly double PFS and 13-fold greater MRD negativity compared to bortezomib monotherapy, but they were also twice as likely to die during the trial compared to monotherapy. When considering the risk-benefit trade-offs of new therapies, such a significant decrease in OS greatly outweighs stellar early efficacy data.
A Joint Effort to Explore Overall Survival
These issues and others surrounding clinical trial endpoints are the focus of FDA’s Project Endpoint, which was launched in 2022 to better understand the benefits and risks of new therapies. In July 2023, FDA, the American Association for Cancer Research (AACR), and American Statistical Association (ASA) collaborated on a workshop that convened broad stakeholders from industry, academia, government, and patient advocacy to discuss best practices and considerations for evaluating OS data moving forward.
This workshop inspired the recent Clinical Cancer Research article that summarizes key considerations for trial designs and drug development strategy, including when to consider OS as the primary endpoint. Importantly, the article encourages trial sponsors to prospectively plan to collect and analyze OS data for every late-phase trial, regardless of whether it is a formal trial endpoint. Additionally, the article details new statistical methodologies to support using OS data to evaluate new therapies for potential harm to patients (see figure), when the data are too limited to be used for efficacy evaluations.
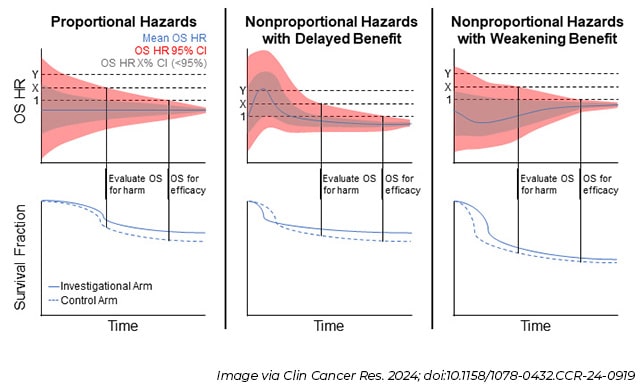
For example, in cases when patients are expected to live for years after taking a therapy, or for rare diseases with limited numbers of patients able to participate in clinical trials, it may take a long time to reach the high-bar definition of “statistical significance,” which could delay access to a new drug. For a finding to be considered statistically significant, researchers must be 95% confident that on average a therapy can extend patients’ lives compared to the standard of care or other control. But what if researchers adopt a less strict approach by evaluating OS for harm, which can be accomplished with fewer patients and in a shorter period?
One possible approach for this would involve being comfortable with a smaller confidence interval (CI) than 95% that the therapy truly extends lives. In the top row of graphs in the above figure, a 95% CI is depicted in red compared to a lower CI in grey. Another strategy is to be 95% confident that a new therapy does not shorten lives more than a certain threshold, which is depicted as the X dotted line in those same graphs. Even if researchers decide to only evaluate OS for harm, it is still important to demonstrate a benefit in other efficacy endpoints, such as tumors shrinking or longer times to progression. Determining fit-for-purpose definitions of harm that include OS, other safety metrics, and patient input ahead of a trial are crucial for clarifying the relationship between early efficacy endpoints and long-term outcomes.
Further, the article examines trial design elements that increase the difficulty of evaluating OS data, such as crossover and unequal randomization, and proposes considerations and strategies to improve the utility of trials that include these elements. The article mentions how OS data are also increasingly important in subgroup analyses in the era of precision medicine to determine which types of patients will receive the most benefit from new therapies. Lastly, the article discusses considerations regarding uncertainty in the benefit-risk profile of new therapies and whether the amount of uncertainty is amenable to accelerated or traditional approval pathways.
While the new paper helps provide clarity on evaluating OS from a harm perspective, more work is needed to validate and advance the use of novel early endpoints. FDA, AACR, and ASA are committed to continuing these efforts with broad stakeholders to maintain the rapid pace of innovation that is providing new treatment options for patients with cancer.
To stay up to date on further developments, subscribe to the free monthly Cancer Policy Monitor newsletter.