Finding Ways to Overcome Ovarian Cancer’s Resistance to Immunotherapy
Nearly a half century ago, one of the founders of cancer immunology, Lloyd J. Old, MD, predicted that “there is something unique about a cancer cell that distinguishes it from normal cells, and that this difference can be recognized by the body’s immune system.” At the time, most of his colleagues disagreed.
But Old was right. Harnessing the body’s natural ability to fight cancer cells turned out to be one of the greatest success stories in medical history. In the decade or so since they’ve become standard of care, cancer immunotherapies have revolutionized treatment. Patients with advanced forms of cancer such as melanoma and non-small cell lung cancer—disease states once associated with short-term survival—have achieved remarkable outcomes, some so long-lasting that patients have been considered cured.
However, Old’s prediction arrived with a qualification. There’s no one single difference that distinguishes a cancer cell from a healthy cell; no centrally located “on-off” switch to activate everybody’s immune system. Instead, there’s a vast, complex, and far-flung network of immune triggers and processes, most of which we don’t fully understand. This could be why some cancers respond well to immunotherapy, while others strongly resist it.
Ovarian cancer, a particularly lethal and difficult-to-treat disease, has thus far been highly resistant to immunotherapies. This is especially frustrating because certain ovarian tumor cells are bad at repairing their own DNA, a weakness known to make other cancers vulnerable to immunotherapeutic targeting.
Fortunately, 2024 saw some new immunotherapy combinations make incremental yet important advances against this malignancy. Let’s take a look at a few.
High Hopes, Low Response
Cancer of the ovaries, the deadliest form of gynecological cancer, has high survival rates if caught early. Unfortunately, it’s usually only diagnosed in its later stages because symptoms are often mild and vague and current screening methods are unreliable. Five-year survival rates of advanced ovarian cancer are below 30%. It is estimated that 19,680 women will be diagnosed with ovarian cancer in 2024 and 12,740 will die from it.
Initial treatment usually involves removing the tumor via cytoreductive debulking surgery combined with platinum-based chemotherapy (such as cisplatin and carboplatin). Only a small percentage of patients with well-differentiated tumors confined to the ovaries will not need additional systemic treatment. This can take various forms.
For example, an aggressive subtype of ovarian cancer called high-grade serous ovarian cancer (HGSOC), usually responds well to chemotherapy given after surgery. But about 20% of HGSOC cases are associated with mutations in the genes BRCA1 or BRCA2, which play a role in a DNA repair pathway called homologous recombination (HR), and ultimately promote cancer growth.
A class of therapeutics called poly (ADP-ribose) polymerase (PARP) inhibitors disrupt the proteins that cancer cells use to fix their DNA. Multiple PARP inhibitors approved by the U.S. Food and Drug Administration (FDA) can treat BRCA-mutation positive and/or HR deficiency-positive ovarian cancers. While these drugs have led to improved outcomes, most patients with ovarian cancer eventually develop resistance and face disease progression.
This is where researchers thought immunotherapy might step in and save the day. Cancers with DNA repair defects tend to be more responsive to therapies that target the immune system. It was hoped a class of drugs called immune checkpoint inhibitors (ICIs) would be effective. Unfortunately, ICIs have never achieved better than a 10% to 15% response rate in ovarian cancer, but researchers continue to develop strategies to overcome this resistance. It could be that we simply haven’t found the right combination therapy yet. After all, researchers point out, in even the most responsive tumor types, such as melanoma and lung cancer, only 20% to 40% of patients respond to single-agent ICIs.
Another factor is probably the state of the tumor microenvironment (TME), which is the ecosystem of cells, molecules and blood vessels that surround and feed a tumor. For example, we know patients with an inflamed, immune-infiltrated TME—so called “hot” tumors—have a better prognosis than those with a TME that is less inflamed or “cold.”
New Combination Therapy
The protein p62 (SQSTM1) plays a role in selective autophagy, signal transduction, inflammatory response, and other processes that occur within a cell. Tumors require p62 to grow and spread, and almost all human tumors contain elevated levels of this protein. Heightened levels of p62 in ovarian cancer cells often indicate poor prognosis and resistance to platinum-based chemotherapies. Recent clinical trial data suggest that targeting p62 could be an effective therapeutic strategy for ovarian cancer.
The trial evaluated Elenagen, an investigational treatment that uses an injectable, supercoiled circular DNA (or plasmid) to promote an immune response that would seek out and destroy cells expressing high levels of p62. The trial enrolled 40 patients with platinum-resistant ovarian cancer, with 20 patients receiving weekly injections of Elenagen plus the chemotherapy drug gemcitabine, and 20 patients receiving gemcitabine alone.
According to results published in Frontiers in Oncology, gemcitabine alone produced an average progression-free survival (PFS) of 2.8 months. But when the chemotherapy was combined with Elenagen, the average PFS was 7.2 months, a statistically significant improvement. Additionally, nine patients in the Elenagen-gemcitabine combination group remained disease free for at least 30 months, the duration of the study.
Finally, the study did not find increased toxicity when Elenagen was added to gemcitabine, an important finding since oncologists often discontinue immunotherapies due to toxicities.
Starving ovarian Tumor Cells
Malignant cancer cells can survive under harsh conditions. But that survival comes at a price. They need more energy and nutrients than healthy cells—and one mineral they need more of is iron.
HGSOC is especially iron dependent, with HGSOC cells showing increased expression of the transferrin receptor, an iron importer, and decreased levels of the iron efflux pump ferroportin when compared to healthy ovarian tissue or even to low-grade serous ovarian cancer.
A paper published in Cancer Discovery, a journal of the American Association for Cancer Research (AACR), in August found that malignant cells located in the ovarian tumor microenvironment overexpressed iron-related gene signatures. Further, the presence of gene signatures involved in transmembrane iron transport across the plasma membrane predicted reduced survival rates among HGSOC patients.
This led the researchers to wonder if disrupting iron accumulation in the ovarian cancer TME might offer a new therapy approach. To test their hypothesis, they treated ovarian cancer mouse models with deferiprone (Ferriprox), an iron chelator (or reducer) approved by the FDA in 2011, both alone and in combination with the platinum-based chemotherapy agent, cisplatin.
Deferiprone alone lowered the number of malignant cells in the peritoneal cavity and reduced the accumulation of ascites, an immunosuppressive peritoneal fluid associated with drug resistance and metastatic disease. The drug slowed the cancer’s spread and decreased tumor-induced enlargement of the spleen. Treated mice showed about 25% increase in median survival over untreated mice. When combined with cisplatin, deferiprone-treated mice experienced about 50% increase in median survival.
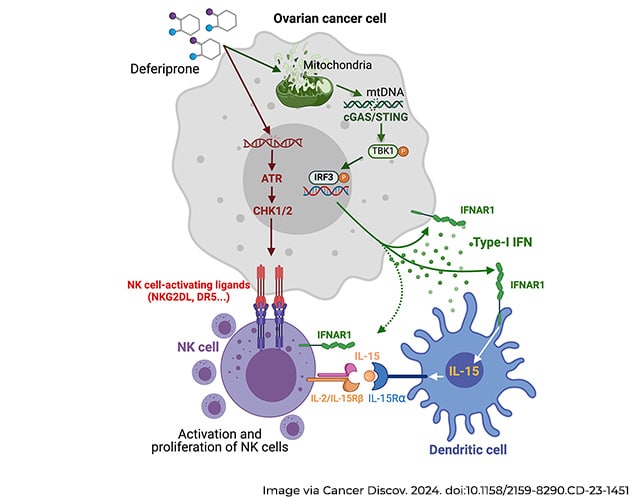
The researchers believe deferiprone works by inducing mitochondrial stress that causes mitochondrial DNA to leak into the cytoplasm, ultimately activating an antitumor immune response that boosts the efficacy of first-line chemotherapy.
FDA-approved, and widely prescribed to patients with transfusional iron overload, deferiprone “might be repurposed as a new immunotherapeutic agent to treat patients with metastatic ovarian cancer,” the paper concluded.
Making Tumors ‘Hot’
A new experimental drug, TILT-123, a virus engineered to attack tumor cells, works by selectively replicating in cancer cells and producing the cytokines tumor necrosis factor alpha and interleukin-2. Viral replication leads to cell lysis, and when the cell ruptures, these cytokines spill out into the TME, causing it to become inflamed, or “hot.” Researchers hope such hot tumors will amplify the strength of ICIs and other immunotherapies in ovarian cancer.
In a phase I clinical trial presented at the AACR Annual Meeting 2024, held April 5-10, 15 patients with ovarian cancer received the new therapy along with pembrolizumab (Keytruda), an ICI. Eight of the 12 evaluable patients showed stable disease, and researchers encountered no dose-limiting side effects. Both injected and non-injected tumors displayed robust immunological activity, and researchers are investigating whether certain immune biomarkers can predict clinical response to the treatment.
Another potential way to inflame tumors is by altering gene expression in the TME. An abstract presented at the same conference looked at tumor samples from 30 patients who participated in a phase II clinical trial evaluating ICI in combination with azacitidine, a chemotherapy drug that also functions as an epigenetic modulator. Researchers examined 72 serial tumor samples collected prior to treatment initiation and after six weeks of therapy found upregulation of inflammatory and cytolytic (or cell destroying) genes. They also found upregulation of the co-inhibitory molecule CTLA-4, in addition to many other inflammatory biomarkers and greater density of tumor-infiltrating immune cells in samples collected during treatment.
Combining an epigenetic modulator and ICI therapy “induces an inflammatory response and reshaping of the tumor microenvironment that may enhance their clinical efficacy,” the authors concluded, “highlighting the therapeutic potential of utilizing epigenetic modulators as a way to sensitize platinum-resistant ovarian cancer to immune checkpoint inhibition.”
The AACR is collaborating with the Rivkin Center to host the 15th Biennial Ovarian Cancer Research Symposium, in Seattle, Washington on September 20-21, for researchers to learn about the latest advances related to ovarian cancer and network with others in the field.