Editors’ Picks, September 2024: Reducing Pancreatic Cancer Risk, Immunotherapy for Dogs, and More
September is nearing an end, which means it’s time for another edition of Editors’ Picks, our monthly staple featuring articles from AACR’s 10 peer-reviewed journals. This month, the editors have highlighted lifestyle factors that may reduce pancreatic cancer risk, a new humanized mouse model to evaluate treatments for prostate cancer, and innovative therapeutic strategies for various solid tumors, among other topics.
Keep reading for the abstracts of the featured studies, and follow the links to the full articles, freely available for a limited time.
Journal: Blood Cancer Discovery
Disruption of KLHL6 Fuels Oncogenic Antigen Receptor Signaling in B-Cell Lymphoma
Pathomechanisms that activate oncogenic B-cell receptor (BCR) signaling in diffuse large B-cell lymphoma (DLBCL) are largely unknown. Kelch-like family member 6 (KLHL6) encoding a substrate-adapter for Cullin-3-RING E3 ubiquitin ligase with poorly established targets is recurrently mutated in DLBCL. By applying high-throughput protein interactome screens and functional characterization, we discovered that KLHL6 regulates BCR by targeting its signaling subunits CD79A and CD79B. Loss of physiologic KLHL6 expression pattern was frequent among the MCD/C5-like activated B-cell DLBCLs and was associated with higher CD79B levels and dismal outcome. Mutations in the bric-a-brac tramtrack broad domain of KLHL6 disrupted its localization and heterodimerization and increased surface BCR levels and signaling, whereas Kelch domain mutants had the opposite effect. Malfunctions of KLHL6 mutants extended beyond proximal BCR signaling with distinct phenotypes from KLHL6 silencing. Collectively, our findings uncover how recurrent mutations in KLHL6 alter BCR signaling and induce actionable phenotypic characteristics in DLBCL.
Significance: Oncogenic BCR signaling sustains DLBCL cells. We discovered that Cullin-3-RING E3 ubiquitin ligase substrate-adapter KLHL6 targets BCR heterodimer (CD79A/CD79B) for ubiquitin-mediated degradation. Recurrent somatic mutations in the KLHL6 gene cause corrupt BCR signaling by disrupting surface BCR homeostasis. Loss of KLHL6 expression and mutant-induced phenotypes associate with targetable disease characteristics in B-cell lymphoma.
Journal: Cancer Discovery
MYC Induces Oncogenic Stress through RNA Decay and Ribonucleotide Catabolism in Breast Cancer

Upregulation of MYC is a hallmark of cancer, wherein MYC drives oncogenic gene expression and elevates total RNA synthesis across cancer cell transcriptomes. Although this transcriptional anabolism fuels cancer growth and survival, the consequences and metabolic stresses induced by excess cellular RNA are poorly understood. Herein, we discover that RNA degradation and downstream ribonucleotide catabolism is a novel mechanism of MYC-induced cancer cell death. Combining genetics and metabolomics, we find that MYC increases RNA decay through the cytoplasmic exosome, resulting in the accumulation of cytotoxic RNA catabolites and reactive oxygen species. Notably, tumor-derived exosome mutations abrogate MYC-induced cell death, suggesting excess RNA decay may be toxic to human cancers. In agreement, purine salvage acts as a compensatory pathway that mitigates MYC-induced ribonucleotide catabolism, and inhibitors of purine salvage impair MYC+ tumor progression. Together, these data suggest that MYC-induced RNA decay is an oncogenic stress that can be exploited therapeutically.
Significance: MYC is the most common oncogenic driver of poor-prognosis cancers but has been recalcitrant to therapeutic inhibition. We discovered a new vulnerability in MYC+ cancer where MYC induces cell death through excess RNA decay. Therapeutics that exacerbate downstream ribonucleotide catabolism provide a therapeutically tractable approach to TNBC (Triple-negative Breast Cancer) and other MYC-driven cancers.
This article was featured on the cover of the September issue.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Background: Pancreatic cancer is among the most fatal human cancers and the fourth leading cause of cancer death in the United States. Evidence suggests that chronic inflammation may play a role in pancreatic carcinogenesis and its inhibition through nonsteroidal anti-inflammatory drugs (NSAID) may reduce pancreatic cancer incidence.
Methods: We examined associations of total and individual NSAIDs with pancreatic cancer risk among postmenopausal women participating in the Women’s Health Initiative observational study and clinical trial cohorts. Among 117,452 women, aged 55 to 79 years, 727 incident pancreatic cancer cases were reported over 18 years of follow-up. Cox regression was used to estimate hazard ratio (HR) and 95% confidence interval (CI) for associations between NSAIDs and pancreatic cancer risk.
Results: Relative to non-use, consistent use of any NSAID was inversely associated with pancreatic cancer risk (HR 0.71, 95% CI, 0.59–0.87), primarily driven by strong associations for aspirin use (HR 0.67, 95% CI, 0.52–0.86). Use of total or individual non-aspirin NSAIDs was not associated with pancreatic cancer. Upon stratified analysis, we observed stronger associations for NSAIDs among participants with prevalent diabetes (HR 0.28, 95% CI, 0.10–0.75) relative to those without (HR 0.75, 95% CI, 0.61–0.92; P-interaction = 0.03).
Conclusions: Additional large prospective studies with careful measurement of NSAID type, dose, and frequency are needed to further investigate the possibility of added benefit among individuals diagnosed with diabetes.
Impact: This study adds to existing evidence from prospective studies and clinical trials suggesting that use of aspirin may provide moderate benefit for pancreatic cancer prevention.
Journal: Cancer Immunology Research
Glioblastoma (GBM) is an aggressive brain tumor with poor prognosis. Although immunotherapy is being explored as a potential treatment option for patients with GBM, it is unclear whether systemic immunotherapy can reach and modify the tumor microenvironment in the brain. We evaluated immune characteristics in patients receiving the anti-PD-1 immune checkpoint inhibitor nivolumab 1 week prior to surgery, compared with control patients receiving salvage resection without prior nivolumab treatment. We observed saturating levels of nivolumab bound to intratumorally and tissue-resident T cells in the brain, implicating saturating levels of nivolumab reaching brain tumors. Following nivolumab treatment, significant changes in T-cell activation and proliferation were observed in the tumor-resident T-cell population, and peripheral T cells upregulated chemokine receptors related to brain homing. A strong nivolumab-driven upregulation in compensatory checkpoint inhibition molecules, i.e., TIGIT, LAG-3, TIM-3, and CTLA-4, was observed, potentially counteracting the treatment effect. Finally, tumor-reactive tumor-infiltrating lymphocytes (TIL) were found in a subset of nivolumab-treated patients with prolonged survival, and neoantigen-reactive T cells were identified in both TILs and blood. This indicates a systemic response toward GBM in a subset of patients, which was further boosted by nivolumab, with T-cell responses toward tumor-derived neoantigens. Our study demonstrates that nivolumab does reach the GBM tumor lesion and enhances antitumor T-cell responses both intratumorally and systemically. However, various anti-inflammatory mechanisms mitigate the clinical efficacy of the anti-PD-1 treatment.
Journal: Cancer Prevention Research
Clonal hematopoiesis (CH) is more common in older persons and has been associated with an increased risk of hematological cancers and cardiovascular diseases. The most common CH mutations occur in the DNMT3A and TET2 genes and result in increased proinflammatory signaling. The Canakinumab Anti-inflammatory Thrombosis Outcome Study (NCT01327846) evaluated the neutralizing anti-IL1β antibody canakinumab in 10,061 randomized patients with a history of myocardial infarction and persistent inflammation; DNA samples were available from 3,923 patients for targeted genomic sequencing. We examined the incidence of non-hematological malignancy by treatment assignment and CH mutations and estimated the cumulative incidence of malignancy events during trial follow-up. Patients with TET2 mutations treated with canakinumab had the lowest incidence of non-hematological malignancy across cancer types. The cumulative incidence of at least one reported malignancy was lower for patients with TET2 mutations treated with canakinumab versus those treated with placebo. These findings support a potential role for canakinumab in cancer prevention and provide evidence of IL1β blockade cooperating with CH mutations to modify the disease course.
Prevention Relevance: We reveal that administering canakinumab is associated with a decrease in non-hematological malignancies among patients with clonal hematopoiesis (CH) mutations. These findings underscore canakinumab’s potential in preventing cancer and provide proof of IL1β blockade collaborating with CH mutations to enhance its clinical benefits.
A related commentary was published in the September issue.
Journal: Cancer Research (September 1 issue)
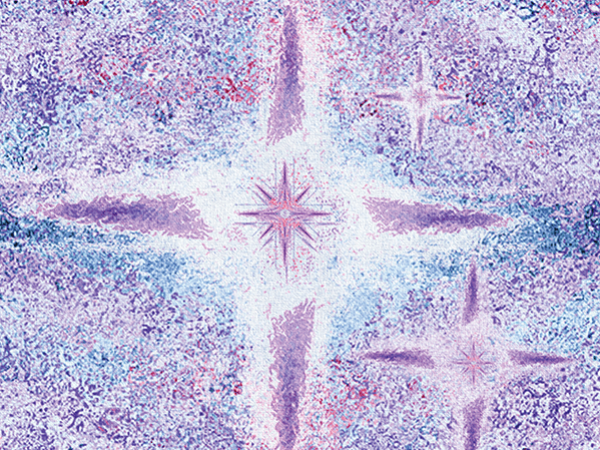
Adaptive metabolic switches are proposed to underlie conversions between cellular states during normal development as well as in cancer evolution. Metabolic adaptations represent important therapeutic targets in tumors, highlighting the need to characterize the full spectrum, characteristics, and regulation of the metabolic switches. To investigate the hypothesis that metabolic switches associated with specific metabolic states can be recognized by locating large alternating gene expression patterns, we developed a method to identify interspersed gene sets by massive correlated biclustering and to predict their metabolic wiring. Testing the method on breast cancer transcriptome datasets revealed a series of gene sets with switch-like behavior that could be used to predict mitochondrial content, metabolic activity, and central carbon flux in tumors. The predictions were experimentally validated by bioenergetic profiling and metabolic flux analysis of 13C-labeled substrates. The metabolic switch positions also distinguished between cellular states, correlating with tumor pathology, prognosis, and chemosensitivity. The method is applicable to any large and heterogeneous transcriptome dataset to discover metabolic and associated pathophysiological states.
Significance: A method for identifying the transcriptomic signatures of metabolic switches underlying divergent routes of cellular transformation stratifies breast cancer into metabolic subtypes, predicting their biology, architecture, and clinical outcome.
This article was featured on the cover of the September 1 issue.
Journal: Cancer Research (September 15 issue)
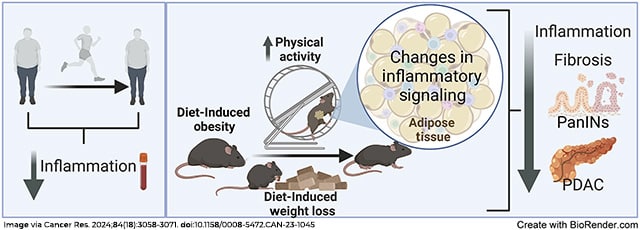
Obesity is a risk factor for pancreatic ductal adenocarcinoma (PDAC), a deadly disease with limited preventive strategies. Lifestyle interventions to decrease obesity represent a potential approach to prevent obesity-associated PDAC. In this study, we examined whether decreasing obesity through physical activity (PA) and/or dietary changes could decrease inflammation in humans and prevent obesity-associated PDAC in mice. Comparison of circulating inflammatory-associated cytokines in subjects (overweight and obese) before and after a PA intervention revealed PA lowered systemic inflammatory cytokines. Mice with pancreatic-specific inducible KrasG12D expression were exposed to PA and/or dietary interventions during and after obesity-associated cancer initiation. In mice with concurrent diet-induced obesity and KrasG12D expression, the PA intervention led to lower weight gain, suppressed systemic inflammation, delayed tumor progression, and decreased proinflammatory signals in the adipose tissue. However, these benefits were not as evident when obesity preceded pancreatic KrasG12D expression. Combining PA with diet-induced weight loss (DI-WL) delayed obesity-associated PDAC progression in the genetically engineered mouse model, but neither PA alone nor combined with DI-WL or chemotherapy prevented PDAC tumor growth in orthotopic PDAC models regardless of obesity status. PA led to the upregulation of Il15ra in adipose tissue. Adipose-specific overexpression of Il15 slowed PDAC growth but only in nonobese mice. Overall, our study suggests that PA alone or combined with DI-WL can reduce inflammation and delay obesity-associated PDAC development or progression. Lifestyle interventions that prevent or manage obesity or therapies that target weight loss–related molecular pathways could prevent progression of PDAC.
Significance: Physical activity reduces inflammation and induces changes to adipose-related signaling to suppress pancreatic cancer, supporting the potential of obesity management strategies to reduce the risk of developing pancreatic cancer.
A related commentary was published in the September 15 issue.
Journal: Clinical Cancer Research (September 1 issue)
Purpose: TILT-123 (igrelimogene litadenorepvec) is an oncolytic adenovirus armed with TNFa and IL2, designed to induce T-cell infiltration and cytotoxicity in solid tumors.
Patients and Methods: TUNIMO (NCT04695327) was a single-arm, multicenter phase I dose-escalation trial designed to assess the safety of TILT-123 in advanced solid cancers refractory to standard therapy. Patients received intravenous and intratumoral TILT-123. The primary endpoint was safety by adverse events (AE), laboratory values, vital signs, and electrocardiograms. Secondary endpoints included tumor response, pharmacokinetics, and predictive biomarkers.
Results: Twenty patients were enrolled, with a median age of 58 years. Most prevalent cancer types included sarcomas (35%), melanomas (15%) and ovarian cancers (15%). No dose-limiting toxicities were observed. The most frequent treatment-related AEs included fever (16.7%), chills (13.0%), and fatigue (9.3%). Ten patients were evaluable for response on day 78 with RECIST 1.1, iRECIST or PET-based evaluation. The disease control rate by PET was 6/10 (60% of evaluable patients) and 2/10 by RECIST 1.1 and iRECIST (20% of evaluable patients). Tumor size reductions occurred in both injected and non-injected lesions. TILT-123 was detected in injected and non-injected tumors, and virus was observed in blood after intravenous and intratumoral injections. Treatment resulted in reduction of lymphocytes in blood, with concurrent lymphocyte increases in tumors, findings compatible with trafficking.
Conclusions: TILT-123 was safe and able to produce antitumor effects in local and distant lesions in heavily pre-treated patients. Good tolerability of TILT-123 facilitates combination studies, several of which are ongoing (NCT04217473, NCT05271318, NCT05222932, and NCT06125197).
A related commentary was published in the September 1 issue.
Journal: Clinical Cancer Research (September 15 issue)
Purpose: Cytokines IL2 and IL12 exhibit potent anticancer activity but suffer a narrow therapeutic window due to off-tumor immune cell activation. Engineering cytokines with the ability to bind and associate with tumor collagen after intratumoral injection potentiated response without toxicity in mice and was previously safe in pet dogs with sarcoma. Here, we sought to test the efficacy of this approach in dogs with advanced melanoma.
Patients and Methods: This study examined 15 client-owned dogs with histologically or cytologically confirmed malignant melanoma that received a single 9-Gy fraction of radiotherapy, followed by six cycles of combined collagen-anchored IL2 and IL12 therapy every 2 weeks. Cytokine dosing followed a 3 + 3 dose escalation design, with the initial cytokine dose chosen from prior evaluation in canine sarcomas. No exclusion criteria for tumor stage or metastatic burden, age, weight, or neuter status were applied for this trial.
Results: Median survival regardless of the tumor stage or dose level was 256 days, and 10/13 (76.9%) dogs that completed treatment had CT-measured tumor regression at the treated lesion. In dogs with metastatic disease, 8/13 (61.5%) had partial responses across their combined lesions, which is evidence of locoregional response. Profiling by NanoString of treatment-resistant dogs revealed that B2m loss was predictive of poor response to this therapy.
Conclusions: Collectively, these results confirm the ability of locally administered tumor-anchored cytokines to potentiate responses at regional disease sites when combined with radiation. This evidence supports the clinical translation of this approach and highlights the utility of comparative investigation in canine cancers.
Learn how clinical trials in companion dogs can inform treatment strategies for human cancers in a recent blog post.
Journal: Molecular Cancer Research
There is tremendous need for improved prostate cancer models. Anatomically and developmentally, the mouse prostate differs from the human prostate and does not form tumors spontaneously. Genetically engineered mouse models lack the heterogeneity of human cancer and rarely establish metastatic growth. Human xenografts are an alternative but must rely on an immunocompromised host. Therefore, we generated prostate cancer murine xenograft models with an intact human immune system (huNOG and huNOG-EXL mice) to test whether humanizing tumor-immune interactions would improve modeling of metastatic prostate cancer and the impact of androgen receptor-targeted and immunotherapies. These mice maintain multiple human immune cell lineages, including functional human T-cells and myeloid cells.
Implications: To the best of our knowledge, results illustrate the first model of human prostate cancer that has an intact human immune system, metastasizes to clinically relevant locations, responds appropriately to standard-of-care hormonal therapies, and can model both an immunosuppressive and checkpoint-inhibition responsive immune microenvironment.
Journal: Molecular Cancer Therapeutics
Radioimmunotherapy (RIT) uses monoclonal antibodies to deliver radionuclides to cancer cells or the tumor microenvironment and has shown promise in treating localized and diffuse tumors. Although RIT agents have gained FDA/EMA approval for certain hematologic malignancies, effectiveness of RIT in treating solid tumors remains limited. In this study, we present PreTarg-it, a novel approach for pretargeted RIT, providing optimized delivery of payloads in a two-step regimen. The effectiveness of PreTarg-it is demonstrated by a powerful combination of ON105, a novel bispecific antibody against both oxidized macrophage migration inhibitory factor (oxMIF) and the histamine-succinyl-glycyl (HSG) hapten, as the first component and the radioactively labeled DOTA-di-HSG peptide as the second component in murine models of cancer. Mice bearing either subcutaneous mouse colorectal CT26 or human pancreatic CFPAC-1 tumors received an IV injection of ON105. After ON105 had accumulated in the tumor and cleared from circulation to approximately 1% to 3% of its peak concentration, 177Lu-DOTA-di-HSG peptide was administered. A single PreTarg-it treatment cycle resulted in tumor regression when mice bearing CT26 tumors were given the highest treatment dose with a pretargeting delay of 3 days. Administered with a 5-day interval, the highest dose arrested tumor growth in both CT26 syngrafts and CFPAC-1 xenografts. In all cases, the highest treatment dose resulted in 100% survival at the study endpoint, whereas the control cohorts showed 0% and 60% survival in the CT26 and CFPAC-1 models, respectively. Therefore, PreTarg-it holds potential as a novel and potent therapy for patients with hard-to-treat solid tumors, such as pancreatic cancer, as well as those with late-stage malignancies.
This article was featured on the cover of the September issue.
Journal: Cancer Research Communications
Precision medicine holds great promise for improving cancer outcomes. Yet, there are large inequities in the demographics of patients from whom genomic data and models, including patient-derived xenografts (PDX), are developed and for whom treatments are optimized. In this study, we developed a genetic ancestry pipeline for the Cancer Genomics Cloud, which we used to assess the diversity of models currently available in the National Cancer Institute–supported PDX Development and Trial Centers Research Network (PDXNet). We showed that there is an under-representation of models derived from patients of non-European ancestry, consistent with other cancer model resources. We discussed these findings in the context of disparities in cancer incidence and outcomes among demographic groups in the U.S., as well as power analyses for biomarker discovery, to highlight the immediate need for developing models from minority populations to address cancer health equity in precision medicine. Our analyses identified key priority disparity-associated cancer types for which new models should be developed.
Significance: Understanding whether and how tumor genetic factors drive differences in outcomes among U.S. minority groups is critical to addressing cancer health disparities. Our findings suggest that many additional models will be necessary to understand the genome-driven sources of these disparities.