Iron in Cancer: Ironing Out the Intricacies
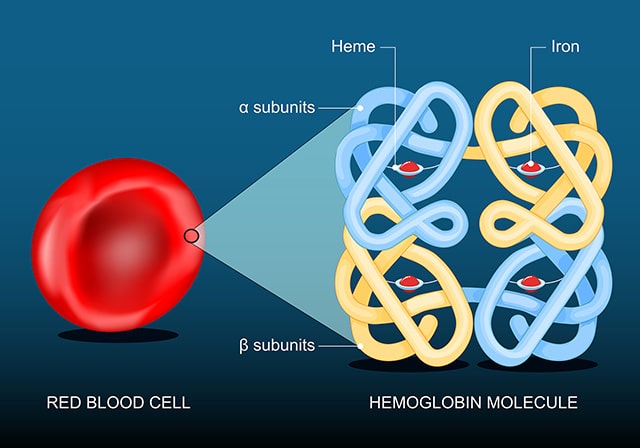
Iron is an essential nutrient best known for its necessity in forming hemoglobin, the protein in red blood cells that transports oxygen from the lungs to distant tissues. It also plays important roles in cell maintenance and growth.
Healthy levels of iron are important to fuel and nourish the normal cells in our bodies, but iron can fuel and nourish cancer cells as well. A flurry of studies in recent years have contributed data, piece by piece, to the complex puzzle of how cancer cells use iron and how iron levels in the body may impact cancer risk.
Studying iron-rich tumors has told researchers a lot about the underlying biology of cancer cells, the immune cells that accompany them, and the stroma that surrounds them. Ironing out the intricacies will be a complicated challenge, fraught with many wrinkles, but it may eventually lead to fresh new therapies for patients.
Excess Iron and Cancer Risk
Because our bodies cannot synthesize iron, we get iron entirely from our diet and supplements. Dietary intake, as well as genetic conditions that can affect how the body absorbs and processes iron, can dictate whether we have too much or too little, which can, in turn, impact overall health.
So, if iron can help cancer cells grow, does having too much iron in your bloodstream affect your risk of cancer?
While the answer isn’t clear cut, some data points to “yes.” One common measurement of healthy iron levels is the amount of an iron storage protein called ferritin circulating in the plasma. Clinical practice has established a range of ferritin levels typically considered “normal,” and ferritin concentrations outside of these ranges may indicate a medical issue that needs to be addressed.
In a study published in Cancer Epidemiology, Biomarkers & Prevention, a journal of the American Association for Cancer Research (AACR), researchers looked at measurements of serum ferritin in over 1 million individuals who received care at a large health system in Israel. They examined the correlation between high ferritin levels—those exceeding three times the upper limit of the reference range—and the risk of developing cancer.
Individuals with elevated ferritin were nearly twice as likely to be diagnosed with cancer as those with ferritin levels below the reference range. The risk was especially high for hematologic malignancies (5.4-fold risk), pancreatic cancer (2.7-fold risk), hepatobiliary cancers (6.4-fold risk), and gastrointestinal malignancies other than colorectal cancer (5.1-fold risk). Conversely, cancers of the breast, male reproductive system, thyroid, brain, and skin were not associated with ferritin levels.
Other researchers investigated whether modulating iron levels could aggravate cases of myelodysplastic syndrome (MDS), a cancer in which the bone marrow makes blood cells that do not mature into healthy blood cells. Some patients with MDS require regular blood transfusions because their red blood cells do not work properly, and regular blood transfusions can put patients at risk of excess iron.
In a mouse model of MDS, researchers overexpressed the iron export protein ferroportin to induce iron overload and found that this worsened several MDS disease characteristics, including more reactive oxygen species and DNA damage and increased numbers of immature myeloid blasts.
Conversely, when the researchers blocked ferroportin with the drug vamifeport, mice with MDS had improved anemia, longer survival, and a lower proportion of immature myeloid blasts than mice that did not receive vamifeport. The researchers suggested that these data may highlight the importance of maintaining healthy iron levels in patients with MDS and may position iron restriction as a potential therapeutic option to help reduce disease severity.
How Cancer Cells Use Iron
At the cellular level, what makes excess iron so lucrative for the development and progression of cancer?
So far, researchers know that too much iron can produce free radicals and reactive oxygen species that can damage DNA, potentially leading to cancer-causing mutations. Iron plays a critical role in the formation of nucleotides that are used to replicate DNA, and it can also activate the cancer driver protein HIF2α.
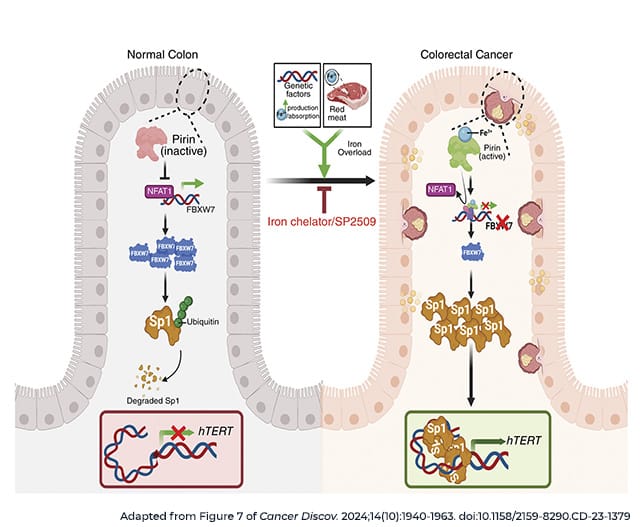
In a recent article in Cancer Discovery, a journal of the AACR, researchers identified an additional mechanism by which iron might promote cancer by helping to elongate telomeres, protective DNA sequences at the ends of chromosomes that can help determine how long a cell survives and how many times it replicates its DNA. To ensure the cell can continue dividing, many cancer cells have mechanisms to elongate their telomeres, including activation of the enzyme telomerase.
Using human colorectal cancer samples, researchers found that iron levels positively correlated with activity of hTERT, a key subunit of telomerase. They observed that iron can activate the iron-sensing protein pirin, which can in turn boost hTERT expression. Further, the researchers identified a small molecule inhibitor of pirin, SP2509, that can block iron-mediated hTERT reactivation. Treatment with SP2509 decreased growth in several cell lines and mouse xenograft models.
Iron is also a crucial component for some types of cellular metabolism, so the ability to process large amounts of iron may help cells grow faster. At the 2024 AACR Special Conference in Cancer Research: Advances in Pancreatic Cancer Research, Joseph Mancias, MD, PhD, an assistant professor of radiation oncology at Dana-Farber Cancer Institute, explained how pancreatic cancer cells accomplish this feat.
Pancreatic cancer cells commonly utilize a process called autophagy, in which cells degrade and recycle intracellular structures. In the course of studying how the cancer relies on autophagy, and perhaps how to target it, Mancias and colleagues identified a protein called NCOA4 that is upregulated during autophagy in pancreatic cancer cells. They showed that NCOA4 can bind to the iron storage protein ferritin and bring it into pockets called autophagosomes, where ferritin is degraded to release free iron. In mouse models, knocking out NCOA4 delayed tumor formation and improved survival.
Mancias expressed hope that the seemingly crucial function of ferritinophagy in pancreatic cancer cells could make it a promising drug target. “[Ferritinophagy] may be one of the most important cell autonomous aspects of autophagy in pancreatic cancer, and it might be worth targeting,” he said.
How Cancer-supporting Cells Use Iron
Cancer cells are not the only cells that can use iron to spur tumor growth. Cancer-associated fibroblasts (CAFs) are a well-characterized component of the tumor microenvironment that can fuel cancer growth and survival in a multitude of ways.
In a recent study, researchers identified a subset of iron-rich CAFs that use their abundant iron to reprogram gene expression so they can drive immune suppression in prostate cancer. These “FerroCAFs” accumulate iron by degrading heme, the iron-containing component of hemoglobin in the blood. The high iron levels activate a genetic reprogramming enzyme called KDM6B, which loosens chromatin and boosts expression of myeloid cell-associated cytokines. The cytokines attract immune-suppressive myeloid cells to the tumor, helping to dampen the antitumor immune response.
Some tumor-promoting immune cells also use iron themselves. Similar to the FerroCAFs, researchers identified an iron-rich population of tumor-associated macrophages (TAMs), another cell type known to contribute to the immune-suppressive microenvironment of many tumor types.
Macrophages can engulf and degrade pathogens as well as infected or damaged cells. This includes red blood cells that have escaped circulation, which can happen when vascular-rich tumors hemorrhage. The researchers showed that TAMs exposed to heme had a unique transcriptional profile with enhanced immune-suppressive capabilities. Inside these iron-rich TAMs (iTAMs), heme can bind to and inactivate the transcriptional repressor Bach1. This allows for the expression of genes like HMOX1, which helps degrade heme to release free iron for the cell to use, and endothelin receptor B (EDNRB).
The researchers characterized EDNRB as a biomarker of iTAMs in mouse sarcoma models and patient samples of synovial sarcoma and melanoma, and also as a driver of iTAMs’ tumor-promoting functions. When they knocked out EDNRB in mouse macrophages, it decreased fibrosarcoma growth and blood vessel infiltration in mice. While more work will be necessary to identify how EDNRB stimulates tumorigenic activity in iTAMs, it may present a viable therapeutic target for further exploration.
Leveraging the Vulnerabilities of Iron-loving Cells
The complex interactions between iron, tumor cells, and the tumor microenvironment will necessitate further research to determine how researchers might best intervene. Nevertheless, a few possible strategies are emerging.
An abundance of iron-related gene signatures among cells in the ovarian cancer tumor microenvironment spurred some researchers to test how iron chelation might affect ovarian cancer progression. In a study published in Cancer Discovery, researchers treated ovarian cancer mouse models with the iron chelator deferiprone (Ferriprox) and observed that the drug decreased tumor growth, the spread of cancer, and the presence of peritoneal micrometastases. Mice that received deferiprone survived a median of 25% longer than untreated mice, a margin that rose to 50% when deferiprone was combined with cisplatin.
Researchers are also investigating how they can use cancer cells’ thirst for iron against them. In recent years, scientists have learned a great deal about a type of iron-dependent cell death called ferroptosis, in which iron oxidizes lipids in the cell, leading to cell death. Often, ferroptosis is precipitated by the genetic, environmental, or pharmacological failure of normal antioxidant mechanisms, leaving the cell unable to undo the oxidative stress caused by the accumulation of iron.
Various research has shown that some cancer cells may protect themselves from ferroptosis by boosting the incorporation of oxidation-resistant lipids, such as oleic acid, into their cell membranes. A few existing therapies, including antiestrogen and antiandrogen hormone therapies for breast and prostate cancer, respectively, can promote ferroptosis by blocking oleic acid incorporation. Other cell signaling mechanisms, including T cell-secreted interferon gamma, can promote the incorporation of pro-ferroptotic lipids into the cell membrane.
As researchers learn more about ferroptosis and how to induce it, the knowledge may provide an additional tool in the growing toolkit of ways researchers can leverage the weaknesses of iron-loving cancer tumors. With iron chelators such as deferiprone already approved for other indications, and several more strategies on the horizon, this field may provide hope for new interventions in coming years.