Head and Neck Cancer: Moving Ahead with Precision Medicine and Immunotherapy
Head and neck cancers encompass a large group of malignancies that originate in the nasal cavity, sinuses, lips, mouth, salivary glands, throat, or larynx. Approximately 90 percent of all head and neck cancers arise in the squamous cells lining the mucosal surfaces of these regions; collectively, these cancers are called head and neck squamous cell carcinoma (HNSCC).
The National Cancer Institute estimates that head and neck cancers account for approximately 4 percent of all cancers in the United States. Survival and mortality statistics for oral cavity and pharynx cancers, a broad category of head and neck cancers, indicate a five-year survival rate of around 65 percent, which has been steadily increasing for the last four decades.
April is Head and Neck Cancer Awareness Month. Read on to learn about categories and risk factors for this disease and a precision medicine approach for treating HNSCC, along with a profile of a metastatic HNSCC survivor who was treated with immunotherapy.
Cancer regions, risk factors, and treatment options
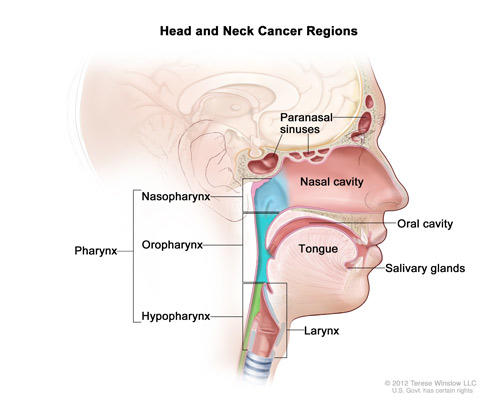
Cancers of the head and neck are delineated based on where the cancer originates. These regions include the oral cavity (including the lips, gums, and the lining of the cheeks); the pharynx (which is further subdivided into the nasopharynx, oropharynx, and the hypopharynx); the larynx (which contains the vocal cords and the epiglottis); the paranasal sinuses and nasal cavity; and the salivary glands.
While some regions have unique risk factors, it is estimated that alcohol and tobacco use account for at least 75 percent of head and neck cancers. While the use of either substance increases the risk for developing head and neck cancers, the combination of both alcohol and tobacco further increases the risk of developing the disease.
Certain viral infections can also increase the risk of certain types of head and neck cancer. For example, infection with the Epstein-Barr virus is a risk factor for nasopharyngeal and salivary gland cancers, and infection with the human papillomavirus (HPV) is associated with increased risk of oropharyngeal cancers. In fact, it is estimated that infection with HPV causes 70 percent of oropharyngeal cancers in the United States.
Overall, head and neck cancers are more common in men, African Americans, and in individuals over age 45. Those of Chinese ancestry are at increased risk for developing nasopharyngeal cancer.
Treatments for head and neck cancers are dependent on the cancer stage and subtype. Therapeutic strategies may include surgery, chemotherapy, radiation therapy, targeted treatment, or a combination of treatments.
Precision medicine: a farnesyltransferase inhibitor for patients with HRAS-mutant HNSCC
Many cancers harbor activating mutations in the oncogene RAS, a key mediator of cellular growth and survival. As such, strategies to inhibit RAS have been a focus in the oncology field for decades. While some drugs aim to inhibit RAS directly, others are focused on inhibiting proteins that modify RAS and are essential for its signaling function. One such modifying protein is farnesyltransferase, an enzyme that catalyzes the addition of a farnesyl group to RAS.
HRAS, one of the three isoforms of RAS, is mutated in roughly 4 percent of HNSCC tumors. This RAS isoform is uniquely sensitive to farnesyltransferase inhibitors.
Last fall, at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, one presentation highlighted results from a phase II clinical trial evaluating tipifarnib, an investigational farnesyltransferase inhibitor, in patients whose recurrent and metastatic HNSCC had progressed on prior therapy.
The study found that treatment with tipifarnib resulted in objective responses in some patients whose tumors had the HRAS gene mutations; the tumors shrunk partially or stopped growing in some of these patients. Notably, no objective responses were seen in these patients on their most recent prior therapy.
“Tipifarnib may represent a promising new therapy for HRAS-mutant HNSCC patients,” said Alan L. Ho, MD, PhD, medical oncologist at Memorial Sloan Kettering Cancer Center and presenter of the study, in an AACR press release. “The success of the trial also speaks to the promise of utilizing genomic sequencing of diseases to identify highly effective therapies that are personalized to the specific biology of each individual patient’s tumor,” Ho noted.
Immunotherapy: a survival story
Bill McCone was diagnosed with HNSCC in 2013. A biopsy revealed that the primary cancer, located in his left tonsil, was caused by HPV.
“I know there’s a lot of negative press out there for the [HPV vaccine], but I would recommend getting the vaccination over what I went through,” said McCone, in an interview with the AACR.
Following his initial treatment, which consisted of six weeks of radiation therapy and weekly infusions of the EGFR inhibitor cetuximab (Erbitux), McCone’s cancer metastasized to his lung. McCone then opted to enroll in a clinical trial which was evaluating the immune checkpoint inhibitor pembrolizumab (Keytruda).
Beginning in October 2014, McCone received an infusion of pembrolizumab every three weeks for two years. While he developed hypothyroidism, which he manages with medication, treatment with pembrolizumab resulted in a complete response for McCone. “Immunotherapy is a real breakthrough,” he said.
See McCone’s story, which was highlighted in the AACR Cancer Progress Report 2017, here:



