Stepping Into the Era of Combination Cancer Therapies, Part 2: Combining Targeted Therapies
In my first post in this series highlighting some of the studies presented at the AACR Annual Meeting 2015 that hold promise for combination cancer therapies, I discussed clinical trials that tested immunotherapy combinations for advanced melanoma. In this post, I discuss a few other clinical trials discussed at the clinical plenary sessions, in which rational combinations of molecularly targeted therapies were tested in subgroups of breast cancer and ovarian cancer. In particular, a couple of clinical trials tested the feasibility of combining olaparib (Lynparza), a poly ADP-ribose polymerase (PARP) inhibitor recently approved by the U.S. Food and Drug Administration for treating advanced ovarian cancer associated with germline BRCA gene mutations, with inhibitors of the PI3K/AKT cell-signaling pathway.
Before getting into the details about the clinical trials that tested the combination, let’s look at how PARP inhibitors and PI3K/AKT inhibitors act against cancer.
What do PARP inhibitors do?
PARP inhibitors, such as olaparib, work against cancer cells based on the principle of synthetic lethality: Cells with a defective BRCA1 gene, a gene responsible for repairing damaged DNA by a process called homologous recombination, can no longer depend on this process to repair their damaged DNA; however, the damaged DNA can be repaired to some extent through another mechanism called base excision repair via poly ADP ribosylation (PAR), which is mediated by the enzyme PARP. Preventing PARP from doing its job using a PARP inhibitor eliminates this option to repair the damaged DNA as well, forcing many cancer cells to die.
What do PI3K/AKT inhibitors do?
The PI3K/AKT/mTOR pathway is one of the most important pathways in cancer metabolism and growth. Dysregulated PI3K pathway signaling is implicated in many types of cancer.
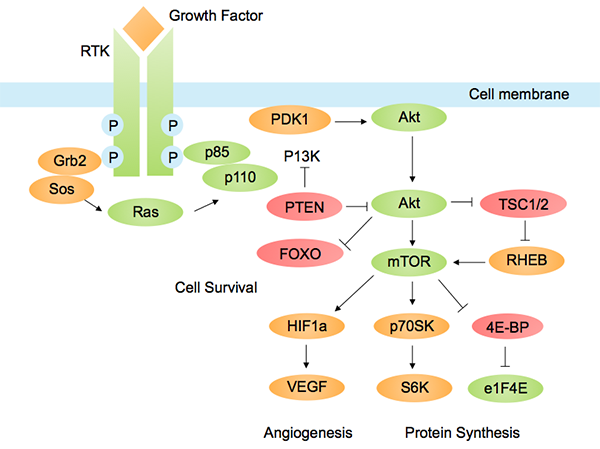
PI3K-Akt pathways with a role in cancer. Image by Tbatan, licensed under CC BY-SA 3.0 via Wikimedia.
In the last couple of years, inhibitors of PI3K and AKT have been developed, which inhibit tumor cell growth and survival in cancer cells dependent on this pathway. Many investigational drugs targeting this pathway are currently being tested in clinical trials.
Why combine them?
While PARP inhibitors and PI3K/AKT inhibitors are effective against cancer when tested as single agents, cancers almost invariably develop resistance to these drugs through multiple mechanisms and find alternate ways to grow. This has prompted researchers to explore rational combinations of drugs that can help circumvent drug resistance.
Combining olaparib with a PI3K inhibitor
In a phase I clinical trial, investigators tested the feasibility of combining olaparib with the PI3K inhibitor BKM120 in patients with high-grade serous ovarian cancer and triple-negative breast cancer.
In earlier studies, investigators of the Stand Up To Cancer (SU2C) “Targeting the PI3K Pathway in Women’s Cancers” Dream Team found that combining olaparib with BKM120 was more effective in mouse models of BRCA-mutant breast cancer and BRCA-wild type triple-negative breast cancer than when either drug was tested individually. So they initiated a phase I clinical trial to test if this combination was feasible and beneficial in patients.
The lead investigator of this trial, Ursula A. Matulonis, MD, director and program leader of medical gynecologic oncology in the Susan F. Smith Center for Women’s Cancers at the Dana-Farber Cancer Institute in Boston, said in a press release, “We are reassured that it is possible to combine olaparib and BKM120 and that we have seen responses in women with triple-negative breast cancer as well as in women with high-grade serous ovarian cancer. It is important that we saw responses against both BRCA-mutant and BRCA-wild type cancers.” She also added, “We need to do further analysis to identify biomarkers that we can use to more effectively identify the patient population that will be most positively affected by the olaparib/BKM120 combination.”
Combining olaparib with an AKT inhibitor
In another phase I clinical trial, called the ComPAKT clinical trial, investigators evaluated if it was possible to safely combine the investigational AKT inhibitor AZD5363 with olaparib for treating advanced cancers, including breast and ovarian cancers associated with defective BRCA genes. Data from this trial showed that it was “indeed possible to combine these drugs safely,” according to lead investigator Timothy Yap, MD, PhD, NIHR BRC clinician-scientist and consultant medical oncologist at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust in London.
Yap said in a press release, “We also observed that several different cancer types responded to the combination, including cancers without BRCA1/2 mutations. These early results are very exciting because preclinical data had suggested that the olaparib and AZD5363 combination had the potential to be effective in a much wider population of patients than just those harboring germline BRCA1/2 mutations.” The team is currently assessing this drug combination in a phase II trial in two different cohorts of patients.
The fact that the studies highlighted here were not the only targeted therapy combinations presented at the meeting and that there were numerous other studies at various stages of investigation underscores the enthusiasm and promise surrounding this approach to cancer treatment.
In my next post in this series, I will discuss how some studies attempted to combine molecularly targeted therapies with hormonal therapies and what these trials taught us.
Cover photo: Dr. Ursula Matulonis presents results of a phase I clinical trial testing the PARP inhibitor olaparib and the investigational PI3K inhibitor BKM120 in breast cancer and ovarian cancer at the AACR Annual Meeting 2015.