Dutch-American Research Team Aiming to Improve Colorectal Cancer Screening
Screening reduces the incidence and mortality of colorectal cancer through the identification and subsequent removal of precancerous lesions and the detection of early-stage cancers, which are more easily treated compared with advanced-stage disease. Despite current screening efforts, colorectal cancer is the fourth most commonly diagnosed cancer worldwide and the fifth leading cause of cancer-related death. A newly formed trans-Atlantic team of researchers brought together by Stand Up To Cancer (SU2C) and the Dutch Cancer Society, KWF Kankerbestrijding (KWF), is hoping to change this by developing a new molecular test for stool-based colorectal cancer screening.
As the Scientific Partner for SU2C, the American Association for Cancer Research (AACR) conducts expert review of the research projects and provides grants management through a rigorous yet nimble, rapid, and transparent process via a “blue ribbon” Scientific Advisory Committee.
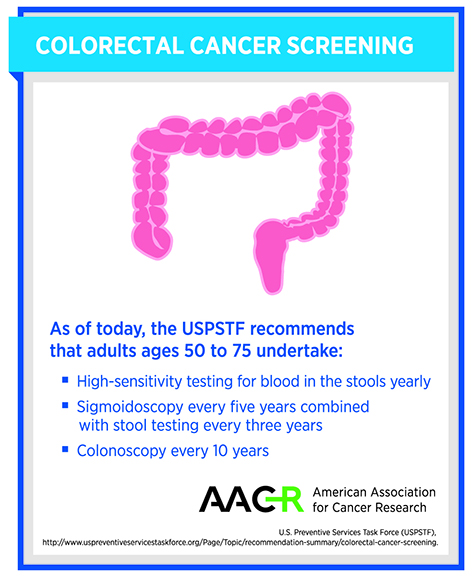
In the United States, the U.S. Preventive Services Task Force (USPSTF) recommends that adults ages 50 to 75 be screened regularly for colorectal cancer. If everyone followed these guidelines, at least 60 percent of U.S. colorectal cancer deaths could be avoided, but unfortunately it is estimated that one in every three U.S. adults for whom screening is recommended is not getting screened.
According to an online survey carried out by the Colon Cancer Alliance in 2011, the number one reason adults age 50 or older gave for not getting screened for colorectal cancer by colonoscopy was fear, with bowel preparation being the hardest part of the procedure.
The only noninvasive colorectal cancer-screening alternative to colonoscopy recommended by the USPSTF is testing stool samples for blood. Research has shown that these tests can reduce colorectal cancer deaths by about 30 percent, but they miss about one-third of cancers and more than two-thirds of precancerous lesions.
As a result, researchers have been working on developing more sensitive stool-based colorectal cancer screening tests for several years. In August, the U.S. Food and Drug Administration (FDA) approved Cologuard, a stool-based test that detects the presence of red blood cells and cancer-associated DNA mutations. While the new test was recently reported to be significantly better at detecting colorectal cancers and precancerous lesions than a standard stool test for blood, it also gave more false-positive results.
Enter the new SU2C and KWF Dream Team, which is hoping to further improve the effectiveness of colorectal cancer screening. Led by Gerrit A. Meijer, MD, PhD, professor of pathology at VU University Medical Center in Amsterdam, with Victor E. Velculescu, MD, PhD, professor of oncology and pathology at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center in Baltimore and member of the AACR Board of Directors, serving as co-leader, the Sta Op Tegen Kanker (Stand Up To Cancer in Dutch) Dream Team will embark on a project titled, SU2C-KWF Molecular Early Detection of Colorectal Cancer.
The Dream Team has two key goals. The first is to improve on current molecular stool-based tests by using the best combination of cancer-associated DNA and/or protein biomarkers, so that these tests can go from the individual level to population screening. The second is to develop a molecular blood-based test that identifies those patients with early-stage disease who are at high risk for disease progression. Such a test would fulfill a tremendous unmet need in treatment of early-stage colorectal cancer by helping to identify those patients who could benefit from chemotherapy after surgery.



