FDA Approvals Bring New Hope for Patients With BRCA-mutant Ovarian Cancer
Just before the holidays, the U.S. Food and Drug Administration (FDA) had good news for the ovarian cancer research community when it announced the approval of both a personalized cancer medicine called olaparib (Lynparza) and a test to identify those patients eligible to receive it: Women with advanced ovarian cancer associated with defective BRCA genes.
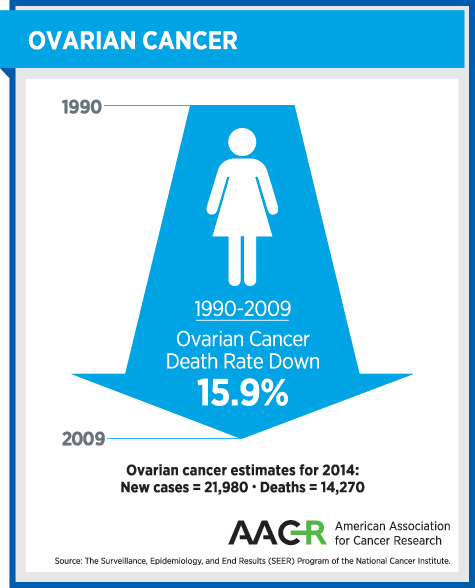
Before this approval, there were no personalized cancer medicines approved for treating ovarian cancer. Progress against the disease was urgently needed, as highlighted by the fact that more than 60 percent of ovarian cancers are diagnosed at an advanced stage, for which the five-year survival rate is just 27 percent.
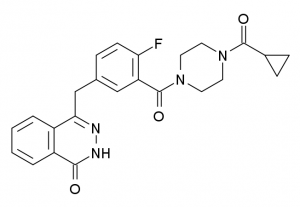
Olaparib is the first in a new class of personalized cancer medicines called poly ADP-ribose polymerase (PARP) inhibitors. It exerts its anticancer activity, at least in part, by blocking the DNA repair function of PARP proteins, which can ultimately trigger cell death.
There are several pathways that can trigger repair of damaged DNA in addition to the base excision repair pathway in which PARP proteins play a role. One of these pathways involves the proteins BRCA1 and BRCA2, and BRCA gene mutations that disable this pathway are estimated to be present in about 15 percent of ovarian cancers diagnosed in the United States. Preclinical studies have shown that cancer cells with one DNA repair pathway out of action as a result of a BRCA mutation are particularly susceptible to blocking of the base excision repair pathway using a PARP inhibitor, and these studies provided much of the rationale for the clinical testing of olaparib.
Olaparib’s December 2014 FDA approval will impact a subgroup of patients with advanced ovarian cancer. Specifically, it has the potential to benefit those who have already been treated with three or more types of chemotherapy and who have a deleterious or suspected deleterious germline BRCA mutation, as detected by a companion diagnostic test called BRACAnalysis CDx, which was approved by the FDA at the same time as olaparib.
The approvals of olaparib and BRACAnalysis CDx were based on initial results from a clinical trial that showed that 34 percent of women with germline BRCA mutation-associated advanced ovarian cancer who received the drug had partial shrinkage or complete disappearance of their tumors for a median of 7.9 months. However, given that the approval centered on response data, rather than overall survival, olaparib’s manufacturer, AstraZeneca, is required by the FDA to conduct a study to confirm that the drug improves survival for patients.
With other PARP inhibitors in development and early evidence that this class of drugs may have activity against other types of cancer that have defects in a DNA repair pathway, for example BRCA mutation–associated breast cancer and some prostate cancers, it is hoped that these drugs will soon benefit patients with a broad array of cancer types.