First FDA-approved Treatment for Rare Form of Non-Hodgkin Lymphoma
Last week, the U.S. Food and Drug Administration (FDA) announced the first-ever approval of a treatment for Waldenström macroglobulinemia, ibrutinib (Imbruvica).
Waldenström macroglobulinemia, which is also known as lymphoplasmacytic lymphoma, is a rare and incurable type of non-Hodgkin lymphoma; about 1,500 individuals are diagnosed with the disease each year in the United States. It arises in immune cells called B cells, or B lymphocytes. As a result, before the approval of ibrutinib, treatment for Waldenström macroglobulinemia was based on approaches approved by the FDA for other, more common types of B-cell lymphoma and leukemia, including chronic lymphocytic leukemia (CLL).
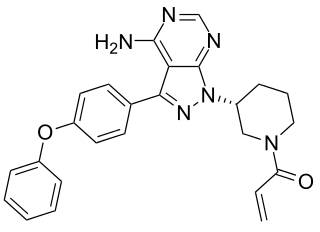
Ibrutinib is a molecularly targeted therapeutic that blocks the function of Bruton agammaglobulinemia tyrosine kinase (BTK), which is a protein that is one component of a signaling pathway that fuels the expansion and survival of Waldenström macroglobulinemia B cells. The signaling pathway also fuels the expansion and survival of a number of other blood cancers that arise in B cells, including CLL and a type of non-Hodgkin lymphoma called mantle cell lymphoma.
In fact, ibrutinib was approved by the FDA for treating patients with mantle cell lymphoma in 2013, and for treating certain groups of patients with CLL in 2014. It is currently being tested in clinical trials as a treatment for several other B cell-originating blood cancers that depend on BTK signaling, including multiple myeloma and diffuse large B-cell lymphoma.
The FDA decision to approve ibrutinib as a treatment for Waldenström macroglobulinemia, which came two months earlier than expected, was based on a phase II clinical trial that showed that 62 percent of patients had their cancer shrink.
One patient with Waldenström macroglobulinemia who has been benefiting from ibrutinib for more than two years is Shelly Lehrman, who was featured in the AACR Cancer Progress Report 2014. Shelly, who was enrolled in a clinical trial testing ibrutinib, says that the molecularly targeted drug allows her to live a life that is as busy and full as it was before her diagnosis. This is a key feature of molecularly targeted therapeutics, which hone in on the Achilles’ heel of a patient’s cancer and are therefore often more effective and less toxic than the treatments that have been the mainstay of patient care for decades. Learn more about Shelly’s story.
http://youtu.be/C3LguUzrtZU