Experts Forecast Cancer Research and Treatment Advances in 2016
Last year was a fruitful year for the cancer research community, which witnessed 16 new approvals and seven new indications by the FDA that included immunotherapies, targeted therapies, and combination therapies. Back in the research laboratories, researchers were making many conceptual advances in making better immune checkpoint inhibitors, CAR T-cell immunotherapies, and targeted therapies, among others, which will shape the future of cancer treatments in the forthcoming years.
And there’s more. Major efforts, like the AACR Project GENIE that launched in November last year, to collect, catalog, and link tumor genetic data with data on patient outcomes with the ultimate goal of empowering health care providers with the knowledge and resources to identify the right treatment for the right patient, were made.
Hopes of making further gains in the field this year soared when the Vice President of the United States, Joe Biden, who lost his son to cancer, promised the nation he would pursue a moonshot to find cancer cures, and lobbied for a major increase in cancer research funding in the government spending bill that Congress passed in December, which resulted in a $2 billion funding increase for the National Institutes of Health, including $264 million for the National Cancer Institute. A panel of 15 members of the AACR—cancer research pioneers and experts—met with the Vice President’s office earlier this month, to discuss strategies that can further his commitment to cancer research.
To top it all, President Barack Obama, in his State of the Union address on Jan. 12, lent his support to Biden’s effort by putting him “in charge of Mission Control.” He said, “Tonight, I’m announcing a new national effort to get it done.” He signed a presidential memorandum today creating a White House task force on cancer, with the goal of doubling the rate of scientific progress toward prevention, treatment, and cure.
We asked experts in cancer precision medicine, immunotherapy, and prevention what breakthroughs we might expect to see in 2016 that would steer the field toward achieving the goals of the vice president. We are finally at the cusp of a transformative phase in which technical advances are further being integrated into cancer clinics, experts say.
Cancer Immunotherapy in 2016
“First of all, it is worth emphasizing that even with all the recent scientific advances and a number of FDA approvals in immunotherapy, we are still just scratching the surface of what can be done,” says cancer immunology and immunotherapy expert Suzanne Topalian, MD, professor of surgery, and director of the melanoma program at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center.
“What I see for 2016 is the following: First, we will continue to gain a lot more knowledge and a deeper understanding through basic laboratory research of how all of the immune pathways work and how drugs can be used to manipulate these pathways to therapeutic advantage,” she says.

Suzanne Topalian, MD, predicts another great year for immunotherapy advances. Photo by © AACR/Scott Morgan 2015.
Second, we will see a continued research emphasis on, and potentially additional FDA approvals for, biomarkers that will allow us to identify patients who are most likely to respond to immunotherapies (companion diagnostics), Topalian speculates. “There is a lot of scientific work connected to ongoing clinical trials to find more sensitive and specific biomarkers, and one possibility is that we may need multifactorial markers,” she says. As an example, when identifying patients most suitable for PD-1 inhibitor therapy, instead of just looking at PD-L1 expression in tumor biopsies, which may exclude some patients who could benefit from this therapy, combining PD-L1 expression results with scoring the presence of certain kinds of immune cells in the tumor may be a better approach, she explains.
Third, Topalian anticipates FDA approval of immune checkpoint inhibitors for more cancer types and as first-line therapy for some. Currently, immune checkpoint inhibitors have been approved as second-line therapy for patients with advanced lung or kidney cancers that have not responded to other therapies; however, ipilimumab (Yervoy), nivolumab (Opdivo), and pembrolizumab (Keytruda), are approved by the FDA as first-line treatment for advanced, unresectable melanoma.
“Time will tell if anti-PD-1 drugs administered even earlier, in adjuvant setting before cancer becomes widespread, will help prolong disease-free survival and overall survival after surgery,” she adds.
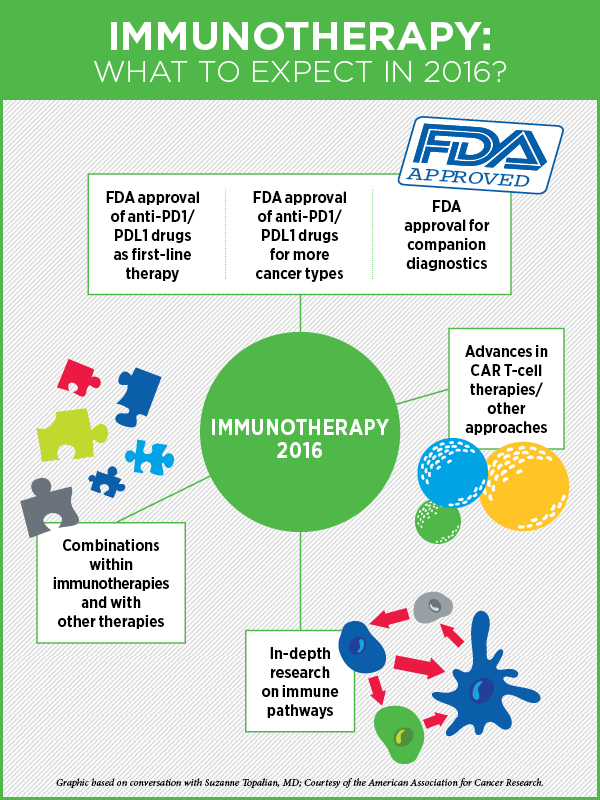
Fourth, Topalian says, we will continue to move towards developing combination treatments that are based on immunotherapies but include another cancer treatment modality such as chemotherapy, targeted therapy, radiation therapy, or epigenetic therapy. Based on data from laboratory models, such combinations are anticipated to be much more potent than using a single modality, she says. A combination of two immunotherapies, nivolumab and ipilimumab, was approved in October last year for melanoma.
Speaking about the prospect of targeted therapies intersecting with immunotherapy, “This is really a growing area of scientific investigation,” Topalian says. “It has become obvious that there are many intersections between genetic events and immunologic events in cancer. I think we can even take it a step further and say that there are intersections with epigenetic events and also with metabolic events. How these intersections can be manipulated to ensure that cancer is eliminated—that is now the focus of cutting edge research,” she adds.
There’s a lot of attention right now on drugs that block the immune checkpoint proteins, but there are other venues for cancer immunotherapy including cancer vaccines, cytokine therapy, and adoptive T-cell transfer, where significant progress is being made, especially in treating advanced cases of leukemia and lymphoma, says Topalian.
“Looking at where we are today, I think there is more reason than ever for cancer patients and their families to be very hopeful,” Topalian notes.
Precision Medicine in 2016
“Cancer precision medicine—individualizing patients’ care based on the specific molecular drivers or other biologic characteristics of their tumor—is now a reality for some patients with many cancer types,” says cancer precision medicine expert David Solit, MD, Geoffrey Beene chair, and director of the Marie-Josée and Henry R. Kravis Center for Molecular Oncology at Memorial Sloan Kettering Cancer Center.
“In lung cancer, in melanoma, and in colon cancer, the optimal treatment is already being dictated by the specific genetic mutations present in a patient’s tumor. But, with the advent of precision medicine–based clinical trials, such as basket trials, where one trial can replace 40 different traditional cancer clinical trials and provide us with answers quicker, I think we’re going to see individualized care becoming the standard for most cancers in the next year or two,” he speculates.
To move the field of targeted therapies forward, an important requisite is to be able to identify the key genetic events that are making the cancer grow in all patients. We are not able to do that right now, Solit says.

Precision medicine expert David Solit, MD, was among the panel of AACR experts that met with the Vice President’s office earlier this month to discuss strategies to further his commitment to cancer research. Photo credit: Memorial Sloan Kettering Cancer Center.
First, the majority of cancer patients in this country have no genetic testing performed whatsoever due to technical reasons or lack of resources, and so they’re not being directed toward rational clinical studies or even the optimal standard therapies, he notes. Second, some insurance companies do not cover such tests. There are also other financial and logistical hurdles, he adds.
But much of this could be overcome by testing for these mutations, not in the tumor tissue, but by using other sources, he says. “For example, can we detect these mutations in the blood?” The use of circulating free DNA collected from blood (commonly referred to as liquid biopsy) to determine which treatment a cancer patient should receive is already a reality, and will begin to change the way we diagnose and treat patients in 2016, Solit says. “In 2016 and 2017, we will likely see liquid biopsies becoming a standard of care for some cancer types.” As the technology for analyzing circulating free DNA is optimized, becomes less expensive, and more widely available, identifying mechanisms of drug resistance is also going to become easier as well, he notes. This should accelerate the development of even more effective targeted agents.
This approach may ultimately save cost as well and spare patients’ exposure to potentially toxic treatments to which they have no possibility of responding, he adds.
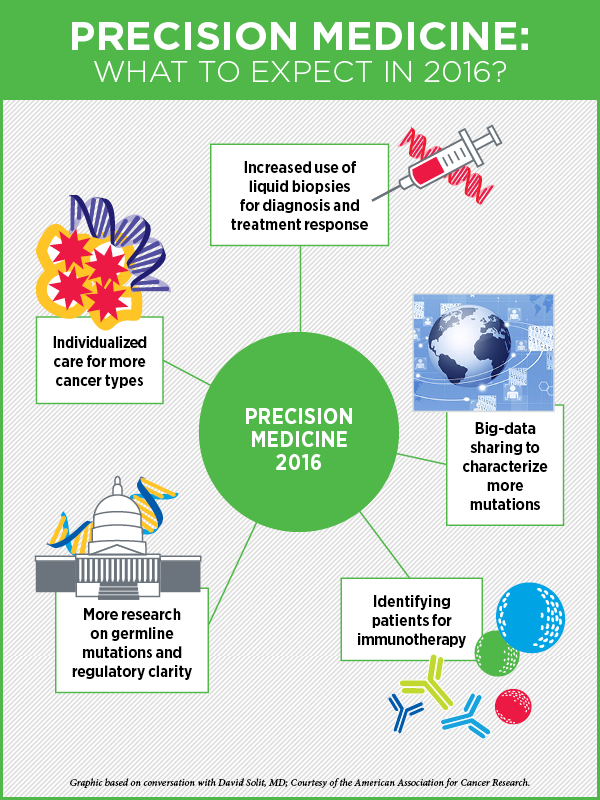
This year, Solit expects to see more effective approaches to big data collection and data sharing. Many institutions have initiated data-sharing programs like the AACR’s Project GENIE, and by pooling data together, for example, many genetic variants of unknown significance will be recognized as recurrent hot spots, facilitating their biological characterization and the clinical testing of drugs against these rare mutations, he explains.
“The other big thing that will happen in 2016 is a greater focus on the identification of germline mutations that are detected as a result of all the tumor testing being performed,” Solit says.
With tumor genetic testing, the initial focus has been to look for somatic mutations (mutations present in the tumor but not in normal cells); however, in this process, germline mutations are often identified as well, which could potentially represent the underlying reason for an individual’s predisposition to the cancer he or she got in the first place, and this could dramatically change the way we approach prevention and screening for different populations, he says.
“We’re going to have to expand our understanding of what such germline mutations mean for patients and their families,” he says, adding that this area is a Pandora’s box right now, because the incidental detection of such germline mutations in patients can impact their children and other family members, brothers and sisters, who may be learning for the first time that they have a mutation that places them as well at great risk of developing cancer.
“I believe that we need significantly more research and more regulatory clarity in this area. Also, every patient may not want to learn at a young age about all the diseases that he or she is at increased genetic risk for in the future given that having a mutation is not a certainty that one will develop the disease,” Solit adds. As our mutational testing expands, these ethical questions are becoming more acute.
Another emerging use of precision medicine is the ability to identify subpopulations of patients for immunotherapy, whose tumors are traditionally thought to be immunotherapy unresponsive, Solit says. An immediate impact will be the search for tumors with microsatellite instability (MSI), he adds. MSI tumors are found most commonly in colon and endometrial cancers, but there are rarer cases of MSI bladder, prostate, esophageal, and gastric cancers, and immunotherapy works incredibly well in many of these patients, he notes.
Cancer Prevention in 2016
“The prospect of refining predictive biomarkers of cancer and, therefore, the ability to better define cancer risks, made possible by precision medicine approaches, has significantly enhanced the field of cancer prevention,” says Fergus Couch, PhD, a cancer prevention expert and professor of laboratory medicine and pathology at Mayo Clinic in Minnesota.
“The first thing we could look for in 2016 is improvements to risk assessment,” Couch says. At present, the age-related risks associated with mutations in cancer predisposition genes in the germline are not well described, explains Couch. Where they are described, confidence intervals are often very wide such that they cannot be used effectively for risk counseling and patient management. “We’re not really certain about how many of these should be presented to the patients,” he says. The goal is to do a better job of selecting patients who can gain some real benefit from knowing the results of their genetic testing, Couch explains, adding that this year he expects to see a lot of studies published in this area that address refinement of risks, particularly for breast, ovarian, and colorectal cancers.
One important step in enhancing risk assessment is to improve the collection of high-quality epidemiological data from patients who are both positive and negative for mutations, says Couch, citing the PROMPT registry as an example, in which patients found to have mutations in any of the predisposition genes are invited to self-enroll in the study through a website. The patients are then followed over time to prospectively collect extensive epidemiological and clinical data.
“We really need the entire community to collaborate on such initiatives” Couch says.
Research on understanding premalignancy for many cancers will be an important area in 2016 for cancer prevention, Couch speculates. “Can we find biomarkers, perhaps, in the blood, that will help us diagnose the progression from a precursor lesion to full cancer?” asks Couch, noting that there are a number of genomic level and targeted DNA and RNA sequencing studies ongoing that are aimed at identifying key pathways and biomarkers that may predict which precursors will advance to become actual cancers.
“I think the infrastructure for the Precision Medicine Initiative of the NIH will be in place by the end of the year. We will see many amazing discoveries coming out of the data from that huge cohort of individuals,” says Couch. This one million-person cohort was initiated with the goal of understanding how we can modify behavior, identify areas that we can change to improve public health, improve risk profiles, and find better ways to improve clinical outcomes and survivorship for patients, he explains.
Earlier this month, the AACR’s journal Cancer Prevention Research published a special report that sets out a brief agenda for the immediate future of cancer prevention research. The report focuses on how the interrelated disciplines of precision medicine and immune-oncology—elements of a broader domain of personalized public health—are transforming cancer prevention, including early detection.
A blog post, written by special report co-author, Scott M. Lippman, MD, director of the UC San Diego Moores Cancer Center, editor-in-chief of the AACR journal Cancer Prevention Research, co-chair of the AACR Cancer Prevention Committee, and member of the AACR board of directors, provides more insight into the report and how we stand on the edge of a new frontier in cancer prevention.
The time is right
With the convergence of incredible technical advances, emergence of strategies to integrate such advances into clinics, and the political will to transform cancer patients’ lives, there’s a lot to look forward to in the field of cancer care this year.


Great piece, very well written and I loved the infographics. One point as a former breast cancer patient: I would have love volunteer for genetic testing but no one at dana Farber could be crystal clear if by participating in genetic testing this could impact health insurances rates. That is a major barrier for patients to agree to be part of such studies. If there is no impact on insurance rates, hospitals need to communicate this proactively.
Great article. Keep up the good work.