AACR Annual Meeting 2017: If You Can’t Drug It, Degrade It – A Protein Degradation Technology to Tackle Undruggable Oncoproteins
Finding a way to therapeutically target the so-called “undruggable” cancer proteins has long been a holy grail of researchers in the field of oncology drug discovery and development.
Advances in research and technology have led to the identification of many molecular targets of cancer to which therapeutics can be directed; however, engaging certain molecules that are known to play a major role in several cancers, such as the transcription factors Myc and Ras, has been formidable for a variety of reasons, including lack of suitable binding pockets for a therapeutic to latch onto and other technical hurdles that make targeting them near-impossible.
About 15 years ago, Craig Crews, PhD, a professor in the Department of Molecular, Cellular and Developmental Biology and in the Department of Chemistry at Yale University, and executive director of Yale Center for Molecular Discovery, sought to address the challenges of dealing with the undruggables by thinking outside the box. Crews pioneered the PROteolysis TArgeting Chimera (PROTAC) technology, a novel approach to degrading disease-causing proteins by exploiting the endogenous protein degradation machinery of cells.
PROTACs are small molecules with two heads – one side of the small molecule binds to the protein of interest and the other side binds to E3 ubiquitin ligase, which plays a role in a cellular process (called ubiquitination) that signals the degradation of the protein. When applied to cancer cells, PROTAC brings the ubiquitin ligase to the proximity of the disease-causing protein, leading to its ubiquitination and subsequent degradation by proteasomes.
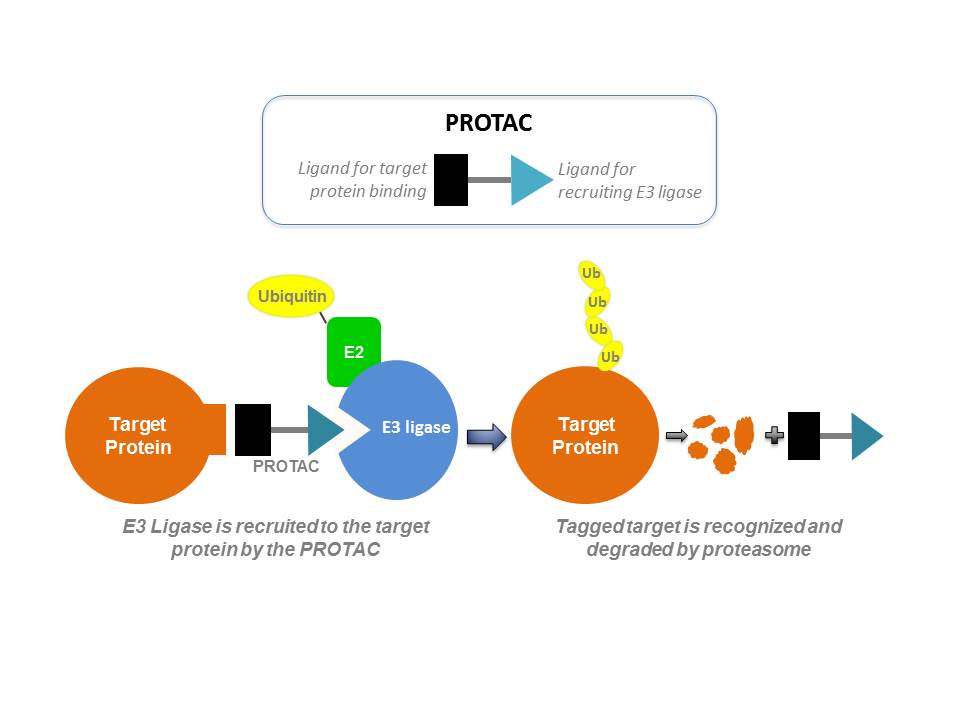 Because degrading a protein with PROTAC technology is not constrained by the requisites of traditional small molecule drug development modalities, it is possible to utilize them to tackle undruggable proteins, according to the researchers developing this technology. “The ability to sub-stoichiometrically degrade transcription factors and scaffolding and other nonenzymatic proteins would greatly increase the current drug target space,” Crews co-wrote in a paper published in Nature Reviews Drug Discovery. “PROTAC generation could become part of the routine strategy in drug discovery, thereby finally making the undruggable proteome pharmaceutically vulnerable.”
Because degrading a protein with PROTAC technology is not constrained by the requisites of traditional small molecule drug development modalities, it is possible to utilize them to tackle undruggable proteins, according to the researchers developing this technology. “The ability to sub-stoichiometrically degrade transcription factors and scaffolding and other nonenzymatic proteins would greatly increase the current drug target space,” Crews co-wrote in a paper published in Nature Reviews Drug Discovery. “PROTAC generation could become part of the routine strategy in drug discovery, thereby finally making the undruggable proteome pharmaceutically vulnerable.”
Crews received the 11th AACR Award for Outstanding Achievement in Chemistry in Cancer Research at the Annual Meeting and, on Tuesday, delivered a lecture titled, “PROTACs: Targeted Protein Degradation as a Therapeutic Strategy.”
In a study presented on Wednesday by Taavi Neklesa, PhD, director of Biology at Arvinas in New Haven, Connecticut — a company that Crews founded— the researchers applied PROTAC technology to develop an orally bioavailable androgen receptor PROTAC (AR PROTAC), and found that it was effective in lowering tumor burden in mice bearing human castration-resistant prostate cancer.
Why a PROTAC to target androgen receptor?
Many men treated with androgen-deprivation therapy as first-line treatment for prostate cancer develop resistance to the therapy over time. Subsequent treatment with enzalutamide (Xtandi) or abiraterone (Zytiga) is not always successful because amplifications and point mutations in the androgen receptor (to which androgens bind) lead to resistance to these therapeutics.
“Because up to two-thirds of patients develop resistance to enzalutamide or abiraterone as the androgen receptor evolves, there is an unmet need that the PROTAC technology can address,” Neklesa said. “The protein degradation technology is well-suited to work in these cases of androgen receptor overexpression and mutations.”
Neklesa and colleagues developed the orally bioavailable AR and tested it in cell lines and animal models. They found that AR PROTAC was very potent, degrading approximately 98 percent of the androgen receptors in the cell lines they tested. The team also observed a dose-dependent inhibition of tumor growth in androgen receptor-amplified VCaP xenograft mice.
AR PROTAC is catalytic
One of the characteristics of resistant prostate tumors is that they start making their own androgens and outcompete the binding of enzalutamide, thus diminishing its efficacy. Because AR PROTAC molecules only need to bind to the androgen receptor transiently to induce degradation, they are capable of overcoming this challenge, Neklesa explained.
“For example, in vitro, if you keep adding androgens to the media containing prostate cancer cells, enzalutamide would not work anymore, but AR PROTACs do, because they don’t need to constantly bind to the androgen receptor, which makes them very effective,” he said.
The team is preparing to test AR PROTAC in patients in a clinical trial soon.
In a post to forecast precision medicine advances in 2017, AACR board member George Demetri, MD, called protein degradation technology “a real game-changer,” and predicted that we would start seeing proof-of-concept studies this year. “I feel it is just about to hit the mainstream,” Demetri noted. It will be interesting to follow the progress in this area.





God bless you. All advances are made “outside the box”. we’ve waited many years for cancer breakthroughs. Millions of sufferers.