How is Obesity Linked to Cancer?
What started the obesity epidemic in the United States? There has been no clear consensus among scientists so far.
Perhaps larger portion sizes shoulder some of the blame? A study found that portion sizes have dramatically increased since the 1970s, and that many common foods exceed the standard portions outlined by the U.S. Department of Agriculture and U.S. Food and Drug Administration.
Maybe our lack of physical activity during the workday has led to weight gain? Research has shown that most jobs of today require less energy expenditure than those 50 years ago.
According to the Centers for Disease Control and Prevention (CDC), obesity is defined as having a body mass index (BMI) ≥ 30, and extreme obesity is defined as having a BMI ≥ 40. Overweight individuals have a BMI ≥ 25. Even though obesity is largely a preventable disease, reports show that obesity rates are increasing worldwide.
Recent statistics concerning obesity in the United States are alarming. Data from the CDC show that the prevalence of obesity nearly tripled from 1960 to 2006 in U.S. adults, and the prevalence of extreme obesity in this population increased over six-fold in this time frame. Recent statistics from the National Institutes of Health indicate that over 70 percent of American adults are overweight or obese.
Perhaps more concerning is the increasing rate of childhood obesity in America, as about 1 in 6 children aged 2-19 are considered obese. A study in the New England Journal of Medicine predicts that over half of today’s children will be obese by the age of 35. Childhood obesity may pave the way for a host of other medical problems, including adulthood obesity and premature death.
Medical risks of obesity include high blood pressure, type 2 diabetes, and cardiovascular diseases. Additionally, obesity has been associated with increased cancer incidence and mortality. To address the link between obesity and cancer, the American Association of Cancer Research is hosting a Special Conference, Obesity and Cancer: Mechanisms Underlying Etiology and Outcomes, this week in Austin, Texas.
We had the pleasure of speaking with cancer epidemiologist and meeting co-chair Elizabeth Platz, ScD, MPH, from the Johns Hopkins Bloomberg School of Public Health about the upcoming meeting. Platz helped to explain some of the mechanisms that surround the link between obesity and cancer, and she highlighted some of the intriguing sessions that will be presented at the meeting.
Beyond carrying excess weight, obesity is comprised of an aberrant physiological state, Platz explained. The disease is associated with insulin resistance, subclinical inflammation, and alterations in adipokines (proteins secreted by adipose tissue, or fat tissue). These characteristics of obesity are associated with cancer.
Platz went on to explain in detail two of the major mechanisms that link obesity and cancer—inflammation and insulin resistance.
How does obesity promote inflammation?
A hallmark of obesity is the substantial expansion of adipose tissue. Our fat cells expand in response to overnutrition, storing the excess calories in the form of triglycerides, which can be released into target tissues at times of energy deficit. The elevated expansion of adipocytes, as occurs in obesity, can lead to cellular damage. This cellular damage initiates a response from the immune system, giving rise to low-level inflammation.
“The immune system is actually quite interesting, because sometimes it can have a non-productive response,” noted Platz. “As a result, you’re left with a smoldering environment. In this environment, free radicals are produced and cause damage to a number of cell types. The immune system doesn’t clear the initial problem, and the inflammatory response continues to cause damage to the local environment, resulting in prolonged subclinical inflammation.”
Platz mentioned that a key element in the link between obesity and inflammation is the ability of adipocytes to produce pro-inflammatory cytokines. These secreted cytokines recruit additional immune cells, facilitating a continual inflammatory microenvironment in those who are obese.
Another important characteristic of expanded adipocytes is the altered levels of the hormones leptin and adiponectin. Leptin, a mediator of inflammation, is responsible for making us “feel full.” Platz explained, “Somehow through the process of becoming obese, the sensitivity to leptin goes away, and those who are obese keep producing more and more leptin, resulting in higher circulating levels.” Additionally, adiponectin is reduced in an obese setting, changing the relative amounts of these key hormones.
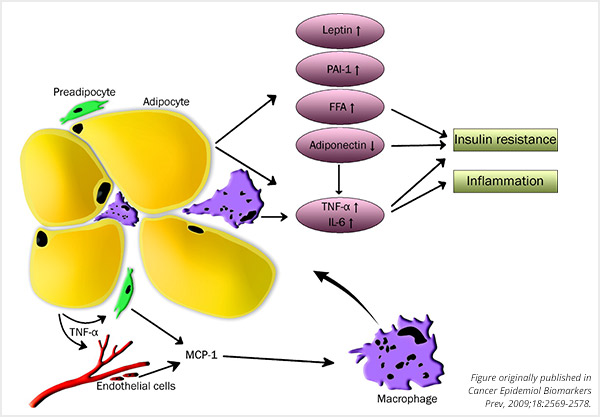
Dysfunctional adipocytes result in inflammation and insulin resistance. TNF-α, tumor necrosis factor- α; MCP-1, monocyte chemoattractant protein; PAI-1, plasminogen activator inhibitor-1; FFA, free fatty acids; IL-6, interleukin-6.
Chronic inflammation can foster the tumor microenvironment, facilitating the replication and survival of cancer cells. In short, our own immune system, which is trying to combat the effects of damaged adipose tissue, can lay the groundwork for cancer cells to grow and thrive.
Can we use this inflammatory response as a target for anti-cancer therapy? One hypothesis is the use of nonsteroidal anti-inflammatory drugs (NSAIDs) to reduce inflammation, but these drugs can have deleterious side effects. “There are side effects to taking NSAIDs—such as gastrointestinal bleeding and brain aneurysm,” noted Platz. “We need to find the right balance between the risks and potential benefits for individuals.”
A plenary session at the conference, titled “Inflammation as a Consequence of Obesity,” will focus on new information on how obesity-induced inflammation plays a role in many cancers.
How does obesity promote insulin resistance?
Interestingly, the location of adipose tissue expansion dictates the probability of insulin resistance, said Platz. While adipocyte expansion in peripheral subcutaneous tissue (fat found underneath the skin) is associated with a decreased risk of insulin resistance, adipocyte expansion in visceral tissue (fat found around the internal organs) is associated with an increased risk of insulin resistance. Compared to subcutaneous adipose tissue, visceral adipose tissue secretes higher levels of the cytokine IL-6 and the protein PAI-1, which are associated with insulin resistance.
How is insulin resistance linked to cancer? When our bodies are resistant to insulin, we have higher amounts of both insulin and glucose in our blood. In addition to mediating blood glucose levels, insulin is also a growth factor, which may foster cancer cell growth, noted Platz. “There’s this complex relationship between glucose and insulin and how they both may be affecting cancer risk,” she explained.
One possible mechanism for insulin resistance leading to cancer is through insulin receptors. Excess insulin can stimulate insulin receptors, which can initiate the MAPK and PI3K protein cascades, two pathways involved in cell growth and proliferation.
Can we combat insulin resistance in cancers? Several investigators are pursuing the use of metformin, a common anti-diabetic drug, as a potential anti-cancer therapeutic, both in observational studies and in clinical trials. Platz, however, noted that this approach has been met with mixed results so far.
What can diabetics and pre-diabetics do to decrease their cancer risk? “Physical activity increases insulin sensitivity,” said Platz. “By exercising and increasing your muscle mass there’s a greater uptake of glucose from circulation and therefore you don’t have to produce as much insulin to keep up.”
In the second plenary session of the conference, titled “Insulin and Glycemia: Roles in Obesity and Cancer,” researchers will discuss what’s new about how insulin resistance affects obesity-related cancers.
Obesity Prevention Strategies
Despite the accumulating evidence, many Americans are not aware that obesity is a risk factor for cancer.
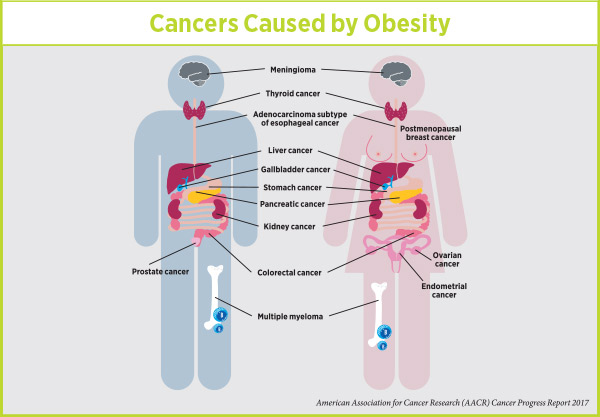
“I don’t know where we went wrong in our messaging,” said Platz. “We got the message across in terms of tobacco use; everybody knows that smoking causes cancer. But Americans don’t seem to be aware that obesity is associated with an increased risk of 14 different cancers.”
 Platz recommends having programs in place to facilitate a healthy lifestyle, especially for children. “Once you gain weight early in life, it’s hard to change your trajectory in adulthood,” she said. School policies and national policies should be focusing on programs for helping those who are overweight to lose weight, she noted.
Platz recommends having programs in place to facilitate a healthy lifestyle, especially for children. “Once you gain weight early in life, it’s hard to change your trajectory in adulthood,” she said. School policies and national policies should be focusing on programs for helping those who are overweight to lose weight, she noted.
“And for adults, we need systems in place to help the overweight population (which unfortunately is most of us) to lose weight, because it’s often hard to do it by yourself,” Platz said. “There are ways that we can intervene now, because obesity is a risk factor for cancer.”
Interested in reading more about the link between obesity and cancer? Read our recent article in Cancer Today, which features an interview with cancer epidemiologist Cornelia Ulrich.