Disrupting a Link Between Obesity and Cancer
The obesity epidemic shows no signs of abating. The World Health Organization estimates that the proportion of the world’s population who are obese nearly tripled between 1975 and 2016, with the proportion of adults age 18 or older who are obese reaching 13 percent in 2016. The prevalence of obesity is even higher in the United States; the most recent data show that 39.6 percent of U.S. adults age 20 and older were obese in 2016. This was up from 37.7 percent in 2014, when another 32.5 percent of adults were classed as overweight.
People who are overweight or obese, as defined by having a high (≥25 kg/m2) body-mass index (BMI), are at increased risk for numerous serious medical conditions, including several types of cancer.
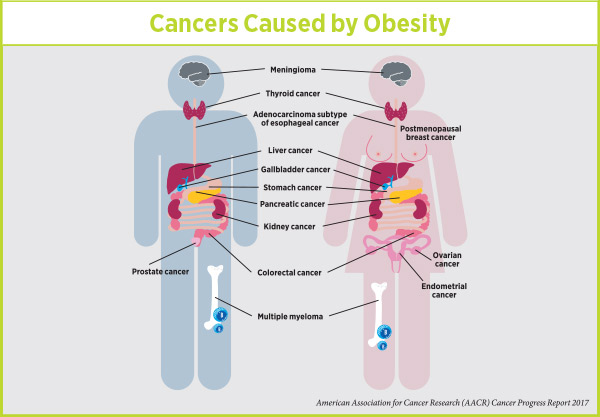
Recently, researchers estimated that high BMI was responsible for more than 544,300 (3.9 percent) of the 14,067,894 new cases of cancer diagnosed worldwide in 2012. Given the current trend of rising obesity rates, the researchers project that the percentage of new cancer cases attributable to high BMI will increase significantly in the coming years.
These grim statistics highlight the need for concerted efforts to develop new clinical, policy, and public education interventions to reduce the prevalence of overweight and obesity, and to reduce the likelihood that those who are overweight or obese go on to develop cancer and other serious medical conditions. The research underpinning many of these efforts was front and center at the recent Obesity and Cancer: Mechanisms Underlying Etiology and Outcomes conference convened by the American Association for Cancer Research (AACR).
As highlighted in a previous post on this blog about the conference, inflammation is one of the main mechanisms believed to link obesity and cancer, and it was the focus of one of the conference’s plenary sessions. During this session, titled “Inflammation as a Consequence of Obesity,” two early-career researchers provided new insight into preclinical studies investigating whether it might be possible to disrupt this link between obesity and cancer with the ultimate goal of reducing cancer risk in people who are overweight or obese.
Targeting inflammation to disrupt a link between obesity and cancer
The researchers, who both work in the laboratory of Stephen D. Hursting, PhD, MPH, at the University of North Carolina at Chapel Hill, set out to investigate whether a nonsteroidal anti-inflammatory drug (NSAID) called sulindac could reduce or overcome the tumor-promoting effects of obesity-associated inflammation using mouse models of two obesity-related cancers—breast cancer and colon cancer.
Postmenopausal breast cancer is one of 14 cancers linked with being obese or overweight. Image courtesy of the National Cancer Institute.
In one set of experiments, MD-PhD student Shannon McDonell and colleagues found that three different breast cancer cell lines generated significantly larger tumors when injected into mice fed a high-fat (obesogenic) diet than if they were injected into mice fed a normal (control) diet. When mice fed a high-fat diet also received sulindac, tumor growth was equivalent to that seen in mice fed the control diet, showing that the NSAID disrupted the effect of obesity on breast tumor growth. Similarly, sulindac also reduced the higher burden of lung metastases seen in obese mice.
In a parallel set of experiments, postdoctoral researcher Laura Bowers and colleagues observed a similar effect in the mouse model of colon cancer. Mice fed a high-fat diet for the duration of the study had significantly more colon tumors than both mice fed a low-fat control diet and mice fed a high-fat diet supplemented with sulindac. Mice fed the control diet and mice fed the high-fat diet supplemented with sulindac had similar numbers of colon tumors.
These preclinical results suggest that NSAID treatment may be able to mitigate the increased risk for cancer caused by obesity. However, as noted by Elizabeth Platz, ScD, MPH, co-chair of the AACR conference in the previous blog post, NSAID treatment can have serious side effects, including gastrointestinal bleeding.
“We need to find the right balance between the risks and potential benefits for individuals,” said Platz.
Given that Bowers and colleagues also found that moderate weight loss disrupted the development of obesity-related tumors in the mouse model of colon cancer, it may be that clinical studies investigating the effects of NSAID treatment on cancer risk should focus on obese individuals who have been unable to achieve weight loss through lifestyle modification, which should remain a central tenet of the efforts to combat the obesity epidemic.




Wow, great post.Really looking forward to read more. Fantastic
Nice post.
Karen isnyour statistic right-numbers dont add to 30%+ of cancer uncidwnce
Hi David, thanks for your good eye. We fixed that error!