AACR Blog Turns 4: How Far Has Immunotherapy Advanced in That Time?
This week marks the fourth anniversary of Cancer Research Catalyst, the official blog of the American Association for Cancer Research (AACR). As outlined in the welcome post from AACR Chief Executive Officer Margaret Foti, PhD, MD (hc), the blog was launched to increase the spread of new knowledge about cancer.
Over these four years, our blog posts have disseminated information about advances across the breadth of cancer research and the clinical cancer care continuum. One area in which advances have occurred at a particularly rapid pace is immunotherapy.
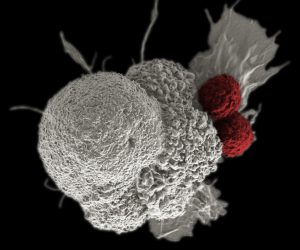
Immunotherapy for cancer works by unleashing the power of a patient’s immune system to fight cancer the way it fights pathogens like the virus that causes flu and the bacterium that causes strep throat. This image is a pseudo-colored scanning electron micrograph of an oral squamous cancer cell (white) being attacked by two cytotoxic T cells (red), part of a natural immune response. Image source: National Cancer Institute\Duncan Comprehensive Cancer Center at Baylor College of Medicine.
Since our first post on immunotherapy, published when the blog was in its infancy, immunotherapy has steadily broken through to the standard of care for an increasing number of cancer types. Thus, it is now firmly entrenched as the fifth pillar of cancer care, together with surgery, radiotherapy, cytotoxic chemotherapy, and molecularly targeted therapy.
This change has been documented on the blog with posts on approvals of new immunotherapeutics and the expansion of immunotherapeutics to treat additional cancer types by the U.S. Food and Drug Administration (FDA). The incredible clinical progress can be boiled down to the fact that when the blog launched on July 1, 2014, there were only five immunotherapeutics approved by the FDA and six types of cancer that could be treated by these agents.
As of July 1, 2018, there were 19 FDA-approved immunotherapeutics. One or more of these agents can be used to treat 19 types of cancer and to treat any type of solid tumor characterized by the presence of a specific molecular signature or biomarker.
The new FDA-approved immunotherapeutics work in various ways to unleash the power of a patient’s immune system to fight cancer. Many are checkpoint inhibitors, which release brakes on the natural cancer-fighting power of the immune system. Others are CAR T-cell therapies, which increase the killing power of the immune system by providing more cancer-targeted T cells. Yet others are therapeutic antibodies that flag cancer cells for destruction by the immune system. The diversity in the way that the new immunotherapeutics work illustrates that we are making progress on many fronts.
But we need to do more, as we have detailed in posts explaining that not all patients have cancers that respond to immunotherapy, and some cancers that respond initially later progress after becoming resistant to the treatments. Identifying ways to expand the scope of immunotherapy so that it can benefit more and more patients is the focus of an increasing number of researchers and has been the topic of many of our posts on immunotherapy research.
The amazing progress made in the field of immunotherapy has certainly captivated our readers. Posts on the topic routinely take multiple spots in our top 10 most read lists. For example, in a post celebrating the third anniversary of the blog, immunotherapy posts accounted for five of the top 10 posts from the prior 12 months. The same was true when we reviewed the top 10 posts of 2017.
Here we list the top 10 immunotherapy blog posts of the past four years, based on page views. We hope you find this a valuable resource that provides a clear overview of progress made in immunotherapy during that time period and the challenges that still need to be overcome.
- Can We Treat Colorectal Cancer With Immunotherapy?
- Lessons Learned From Combining Anti-OX40 and Anti-PD1 Immunotherapies
- Advances in Immunotherapy: Fine-tuning CAR T Cells
- FDA Approves First Immunotherapy-Companion Diagnostic Combo for Lung Cancer
- How Cancers Lose Something to Gain Immunotherapy Resistance
- AACR Annual Meeting 2017: Immunotherapy Provides Long-lasting Responses to Certain Cancer Types
- Why Does Immunotherapy Not Benefit Everyone Long-term?
- FDA Approves First Immunotherapy for Children With Cancer
- Groundbreaking Cancer Immunotherapeutic Approved by FDA
- Top Trend in Health Care in 2015: Cancer Immunotherapy