FDA Approves New Treatments for Several Rare Cancers
In the midst of the dog days of summer, the U.S. Food and Drug Administration (FDA) has provided good news for the cancer community: It has approved two new anticancer therapeutics for the treatment of four rare types of cancer. On July 30, 2018, it approved a targeted radiotherapeutic—iobenguane iodine (I) 131 (Azedra)—for treating certain patients with two types of neuroendocrine tumors, pheochromocytomas and paragangliomas. Then, on Aug. 8, 2018, it approved an immunotherapeutic—mogamulizumab-kpkc (Poteligeo)—for treating certain patients with two types of non-Hodgkin lymphoma, mycosis fungoides and Sézary syndrome.
First FDA-approved treatment for pheochromocytomas and paragangliomas
Pheochromocytomas and paragangliomas are rare types of neuroendocrine tumors. Most patients have localized tumors that are presumed to be benign, but approximately 100 to 200 people in the United States are estimated to be diagnosed with malignant forms of these tumors each year. In about 25 percent of cases, pheochromocytomas and paragangliomas develop in people who have an inherited cancer-associated mutation, according to the National Cancer Institute (NCI).
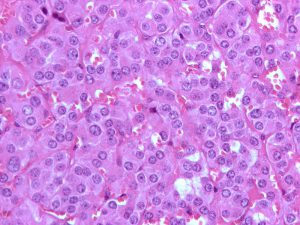
Micrograph of a pheochromocytoma, a rare type of neuroendocrine tumor. Image by Nephron, via Wikipedia.
Pheochromocytomas and paragangliomas arise from cells in the adrenal glands, and cells along nerve pathways in the head, neck, and spine, respectively. In many cases, the cells in which these tumors originate secrete catecholamines, hormones that include epinephrine and norepinephrine. The secretion of large amounts of these hormones by these tumors can cause elevated blood pressure, palpitations, sweats, and anxiety.
Iobenguane, which is also known as metaiodobenzylguanidine (MIBG), is a molecule that is analogous to norepinephrine. It targets the norepinephrine transporter, which is present on the surface of norepinephrine-secreting pheochromocytomas and paragangliomas. Imaging using iobenguane labeled with either of the radionuclides I-123 or I-131 has been used to locate pheochromocytomas and paragangliomas in the body for cancer diagnosis and treatment monitoring since the early 1980s.
Azedra is a new version of iobenguane I-131 that delivers more radiation to tumors than the version used for imaging because of the technology used to generate it. The technology helps eliminate iobenguane that is not labeled with I-131 from being present in the final product, making Azedra a highly active targeted radiotherapeutic.
The FDA approval of Azedra is for the treatment of patients age 12 and older with locally advanced or metastatic pheochromocytoma or paraganglioma whose tumors test positive for the norepinephrine transporter using iobenguane imaging. The approval was based on results from a phase II clinical trial that showed that 25 percent of patients met the trial’s primary endpoint, which was a 50 percent or greater reduction in their use of medications to treat high blood pressure for at least six months.
One of the trial’s secondary endpoints was overall tumor response, and 22 percent of patients had either a partial or complete response.
A new immunotherapeutic for two cutaneous T-cell lymphomas
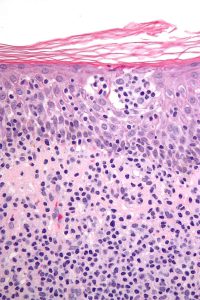
Micrograph of cutaneous T-cell lymphoma. Cutaneous T-cell lymphomas are a group of rare non-Hodgkin lymphomas that includes mycosis fungoides and Sézary syndrome. Image by Nephron via Wikipedia.
Cutaneous T-cell lymphomas are types of non-Hodgkin lymphoma that arise in immune cells called T cells. In these diseases, the cancerous T cells accumulate in the skin, resulting in an itchy, red rash.
Cutaneous T-cell lymphomas are rare types of cancer; researchers estimate that the incidence of these diseases in the United States is about 6.4 cases per million persons. Mycosis fungoides and Sézary syndrome account for about two-thirds of the cases of cutaneous T-cell lymphomas.
In many cases, the cancerous T cells in patients with mycosis fungoides or Sézary syndrome have a protein called CCR4 on their surface. The newly approved immunotherapeutic mogamulizumab-kpkc targets CCR4. Once mogamulizumab-kpkc attaches to CCR4 on the surface of the cancerous T cells, it flags the cancer cells to immune cells, which upon attaching to another part of mogamulizumab-kpkc are triggered to destroy the cancer cells.
The FDA approval of mogamulizumab-kpkc is for the treatment of adults who have mycosis fungoides or Sézary syndrome that has not responded to or has relapsed after treatment with at least one other systemic therapy. The approval was based on results from the MAVORIC phase III clinical trial, which were published recently in Lancet Oncology. In brief, the results showed that mogamulizumab-kpkc more than doubled progression-free survival for patients with relapsed or refractory mycosis fungoides or Sézary syndrome compared with vorinostat (Zolinza), which is a standard treatment in this clinical setting; the median progression-free survival was 7.6 months among those who received mogamulizumab-kpkc versus 3.1 months among those who received vorinostat.
More progress is needed against rare cancers
For three of the rare cancers discussed in this post—pheochromocytomas, paragangliomas, and Sézary syndrome—these FDA approvals mark the first time the agency has approved a drug specifically to treat these diseases. This highlights the need for more research into all types of cancer, including those that affect just small numbers of individuals.
Editor’s note: You can read about all the FDA approvals made from Aug. 1, 2017, to July 31, 2018, in the AACR Cancer Progress Report 2018, which will be released Sept. 12, 2018. The report will be freely available at cancerprogressreport.org, where you can also find the previous seven editions of the annual report.