Recent FDA Approvals Signal More Progress Against Cancer
The past month has seen the U.S. Food and Drug Administration (FDA) expand the use of four anticancer therapeutics and approve a new one, providing additional treatment options for patients with four types of cancer. On Aug. 16, 2018, the agency approved the immunotherapeutic nivolumab (Opdivo) for treating certain patients with small cell lung cancer and approved the molecularly targeted therapeutic lenvatinib (Lenvima) for treating certain patients with the most common type of liver cancer, hepatocellular carcinoma. On Aug. 27, 2018, it approved a combination of the molecularly targeted therapeutic ibrutinib (Imbruvica) and the immunotherapeutic rituximab (Rituxan) for treating a rare form of non-Hodgkin lymphoma called Waldenström’s macroglobulinemia. Then, on Sept. 13, 2018, it approved the new molecularly targeted therapeutic moxetumomab pasudotox-tdfk (Lumoxiti) for treating a rare type of leukemia called Hairy cell leukemia.
Use of nivolumab expanded to new type of lung cancer
Lung cancer is the second most commonly diagnosed cancer in the United States, with 234,030 new cases expected in 2018, according to data from the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results Program (SEER). There are two main types of lung cancer: non–small cell lung cancer (NSCLC), which accounts for up to 85 percent of lung cancers, and small cell lung cancer (SCLC), which accounts for up to 15 percent of cases.
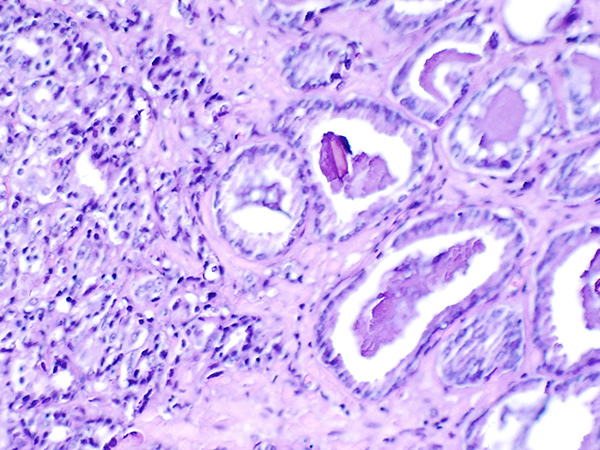
Histopathologic image of small cell lung cancer, one of the two main types of lung cancer. Image via Wikipedia.
The prognosis for patients diagnosed with small cell lung cancer is poor; the five-year relative survival rate is just 5 percent to 10 percent, according to the NCI. For those with metastatic disease the outlook is even worse; median survival is just six to 12 months with the treatments available before the approval of the immunotherapeutic nivolumab.
Nivolumab was approved for patients with metastatic small cell lung cancer that has progressed after platinum-based chemotherapy and at least one other treatment. According to the FDA statement, the approval was based on data from a subgroup of patients enrolled in the phase I/II CheckMate-032 clinical trial who had metastatic SCLC. Among 109 patients whose SCLC had progressed after platinum-based chemotherapy and at least one other treatment, nivolumab led to partial or complete tumor shrinkage in 13, which is 12 percent. The responses lasted six months or longer for 77 percent of these 13 patients and 12 months or longer for 62 percent.
As discussed in earlier posts on this blog, prior to this approval, nivolumab had already been approved for treating certain patients with NSCLC and seven other types of cancer: bladder cancer, colon cancer, head and neck cancer, Hodgkin lymphoma, kidney cancer, liver cancer, and melanoma.
Nivolumab has broad utility in the treatment of cancer because it does not target the cancer directly, rather it works by releasing a brake called PD-1 on cancer-fighting immune cells called T cells. Nivolumab releases the PD-1 brake by preventing it from being engaged by two proteins called PD-L1 and PD-L2. Once the PD-1 brake is released, the T cells can destroy cancer cells.
A new antiangiogenic treatment option for liver cancer
Liver cancer incidence is rising in the United States. Depicted here are nanoparticles in a liver tumor. Image courtesy of the National Cancer Institute.
Liver cancer incidence has been increasing in the United States for the past four decades, according to data from the NCI SEER Program. New approaches to treatment are urgently needed, as the five-year relative survival rate has improved little during that time; it remains at just 17.7 percent.
The majority of the 42,220 new cases of liver cancer expected to be diagnosed in the United States in 2018 will be classed as hepatocellular carcinoma. Many patients diagnosed with advanced hepatocellular carcinoma are treated with sorafenib (Nexavar).
Sorafenib exerts anticancer effects in several ways because it targets a number of molecules called tyrosine kinases that promote growth and cancer progression in different ways. Some of these molecules promote the growth of the blood and lymphatic vessel networks that tumors establish to grow and survive. Thus, one effect of sorafenib is to impede the growth of new blood and lymphatic vessels. As such, it is known as an antiangiogenic therapeutic.
Lenvatinib is also an antiangiogenic therapeutic. Its approval as an initial treatment for patients who have hepatocellular carcinoma that cannot be treated with surgery was based on results from the phase III REFLECT clinical trial. The results, which were published in the Lancet, showed that lenvatinib was not inferior to sorafenib in overall survival, nor was it statistically significantly better; median overall survival was 13.6 months among the 478 patients who received lenvatinib compared with 12.3 months among the 476 patients who received sorafenib.
As discussed in earlier posts on this blog, prior to this approval, lenvatinib had already been approved for treating certain patients with thyroid cancer and kidney cancer.
A new combo of a molecularly targeted therapeutic and an immunotherapeutic for non-Hodgkin lymphoma
3D rendering of a B cell, the type of immune cell in which Waldenström’s macroglobulinemia arises. Image by Blausen Medical via Wikipedia.
Waldenström’s macroglobulinemia, which is also known as lymphoplasmacytic lymphoma, is a rare and incurable type of non-Hodgkin lymphoma; about 1,100 individuals are diagnosed with the disease each year in the United States, according to the NCI. It arises in immune cells called B cells.
Ibrutinib became the first treatment approved by the FDA specifically for patients with Waldenström’s macroglobulinemia in January 2015. Before that, treatment for Waldenström macroglobulinemia was based on approaches approved by the FDA for other, more common types of B-cell non-Hodgkin lymphoma, including the use of the immunotherapeutic rituximab.
Ibrutinib and rituximab both target B cells. Ibrutinib is a molecularly targeted therapeutic that blocks the function of Bruton agammaglobulinemia tyrosine kinase (BTK), which is a protein that is one component of a signaling pathway that fuels the expansion and survival of Waldenström macroglobulinemia B cells. Rituximab targets CD20, a protein on the surface of Waldenström macroglobulinemia B cells. After it attaches to CD20, rituximab flags the Waldenström macroglobulinemia B cells for destruction by immune cells.
Given that both ibrutinib and rituximab have yielded responses for some patients with Waldenström macroglobulinemia, researchers decided to test whether combining the therapeutics might improve outcomes for patients.
The approval of the ibrutinib/rituximab combination for patients with Waldenström macroglobulinemia was based on results from the phase III iNNOVATE clinical trial, according to the developer of ibrutinib, AbbVie. The results, which were published recently in The New England Journal of Medicine¸ showed that 30 months after starting treatment, the progression-free survival rate was 82 percent among those patients who received the ibrutinib/rituximab combination versus 28 percent among those who received a placebo and rituximab.
A new molecularly targeted therapeutic for hairy cell leukemia
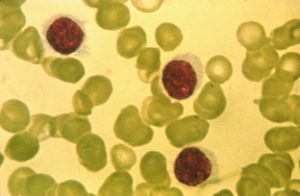
The name hairy cell leukemia stems from the “hairy” appearance of the leukemia cells under a microscope. Image courtesy of the National Cancer Institute.
Hairy cell leukemia is a rare type of leukemia; researchers recently reported that there are about 1,000 new cases diagnosed each year in the United States. Many patients are treated with the cytotoxic chemotherapeutic cladribine. This often leads to durable remission, which can last 10 years or more. However, up to 50 percent of patients eventually relapse and new approaches to treating these patients are urgently needed.
Given that hairy cell leukemia arises in immune cells called B cells, researchers have been investigating whether targeting proteins found on B cells might provide a good therapeutic strategy. One B cell protein that is found on hairy cell leukemia cells is CD22.
Moxetumomab pasudotox-tdfk targets CD22. The new molecularly targeted therapeutic is known as a recombinant immunotoxin because it comprises a CD22-targeting portion fused to a bacterial toxin that causes cell death. Once the CD22-targeting portion attaches to CD22 on the surface of hairy cell leukemia cells, the therapeutic is internalized by the cell where the toxin is released and causes the leukemia cell to die.
According to the statement from the FDA, moxetumomab pasudotox-tdfk is approved for treating patients with hairy cell leukemia who need a new treatment after having tried two previous treatments, including cladribine or another cytotoxic chemotherapeutic that works in the same way. The approval was based on results from the 1053 phase III clinical trial, which were published recently in Leukemia. These results showed that 30 percent of the 80 patients who received moxetumomab pasudotox-tdfk had a durable complete response, which was defined as maintenance of hematologic remission for more than 180 days after reaching a complete response. Overall, 75 percent of the patients had a partial or complete response.
Looking to more progress in the future
As highlighted last week in the AACR Cancer Progress Report 2018, advances against cancer like those discussed in this blog are rooted in decades of basic research. As research continues to deepen our understanding of the complexities of cancer, we can expect to see many new anticancer therapeutics developed in the future and further expansion in the use of those already in the clinic.