AACR Journals Editors’ Picks for February
As a regular post on this blog, we feature the 10 articles chosen by our editors from all journal issues published each month by the American Association of Cancer Research (AACR). For February, these articles span from a review of recent preclinical studies focused on brain metastases to a first-in-human immunotherapy trial. As always, articles highlighted here are freely available for a limited time.
Journal: Cancer Research (February 1 issue)
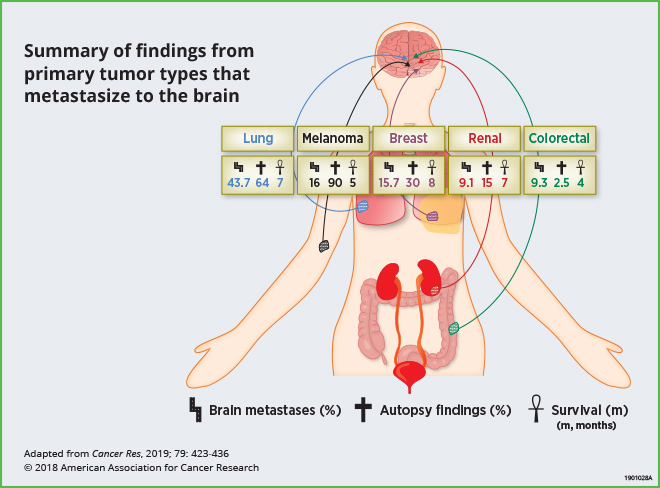
A Blazing Landscape: Neuroinflammation Shapes Brain Metastasis
Up to 30 percent of adult patients with systemic malignancies develop brain metastases, which bear poor clinical outcomes. Current treatments have limited efficacy, resulting in a median survival of less than one year. The most common cancers that metastasize to the brain are lung, melanoma, breast, renal, and colorectal cancers. This review highlights recent preclinical research that has analyzed the brain microenvironment and neuroinflammation; these studies indicate that reciprocal interactions between tumor cells and brain stromal cells facilitate metastatic growth. The authors point out that while clinical studies that target components of the tumor microenvironment in this setting are still limited, the integration of preclinical findings described here may help facilitate novel clinical strategies for the prevention and treatment of brain metastases.
Journal: Clinical Cancer Research (February 1 issue)
While checkpoint immunotherapy has revolutionized the treatment of patients with non-small cell lung cancer (NSCLC), a subset of patients develop hyperprogressive disease, yet the pathological features or underlying mechanisms for this association have yet to be fully understood. In this study, the authors evaluated 187 NSCLC patients treated with checkpoint blockade immunotherapy at a single center in Milan; of 152 evaluable patients, 39 (25.7 percent) developed hyperprogressive disease, all of which showed tumor infiltration by M2-like CD163+CD33+PD-L1+ clustered epitheloid macrophages in pretreatment samples. In preclinical models utilizing human lung cancer cells and specific NSCLC mutational subsets, the researchers found that tumor growth was enhanced by anti-PD-1 treatment but not by anti-PD-1 F(ab)2 fragments, which lack the Fc portion of the antibody. These results suggest that Fc/FcR triggering by immune checkpoint blockade can reprogram macrophages to have aggressive, protumorigenic characteristics and indicate that innate immunity may play a role in hyperprogressive disease.
Journal: Cancer Prevention Research
The preventative effect of finasteride, an inhibitor of the critical androgen metabolism enzyme 5α-reductase, was evaluated in the Prostate Cancer Prevention Trial (PCPT), and the results were mixed. To investigate if specific genotypes alter the effect of finasteride on high-grade cancer risk, the authors evaluated germline genetic data from 1,157 prostate cancer cases in PCPT. After analyzing 357 single nucleotide polymorphisms (SNPs) from 83 candidate genes, the authors found that men carrying a GG genotype in the SRD5A1 gene at locus rs472402 had a 55 percent risk reduction in developing high-grade prostate cancer when taking finasteride. Additionally, the authors built a prediction model including 28 SNPs which classified 37 percent of men enrolled in PCPT to have reduced risk of high-grade cancer when taking finasteride.
Journal: Molecular Cancer Research
Treatment with VEGF inhibitors can delay progression in pancreatic ductal adenocarcinoma (PDA), however, many patients either do not respond to this treatment or eventually relapse. Utilizing agents that enhance the efficacy of anti-angiogenic therapies is a potential treatment strategy for this hard-to-treat cancer. In this study, the authors evaluated the dual inhibition of VEGF and cyclooxygenase-2 (COX-2), a potential mediator of VEGF-independent tumor angiogenesis, in PDA models. The combinatorial treatment substantially limited metastases compared to inhibition of VEGF or COX-2 alone, through reduced collagen deposition, reversed epithelial-mesenchymal transition, and altered immune landscape through the modulation of T-cell subpopulations. These results support the rationale for dual VEGF and COX-2 inhibition in PDA patients and suggest that this approach may enhance immunotherapeutic strategies.
Journal: Cancer Discovery
EIF1AX and RAS Mutations Cooperate to Drive Thyroid Tumorigenesis through ATF4 and c-MYC
Eukaryotic initiation factor 1A (EIFIA), which is encoded by the EIFIAX gene, is required for the assembly of the 43S pre-initiation complex (PIC), an essential component of protein translation. Mutations in EIFIAX often coincide with RAS gene mutations in poorly differentiated and anaplastic thyroid cancers. In this study, the authors found that EIFIAX-A113 splice variants stabilize the PIC and preferentially translate ATF4, which leads to a general increase in protein synthesis. Additionally, EIFIAX and RAS mutations were found to stabilize c-MYC, which promoted mTOR activation and generated therapeutic vulnerabilities to inhibitors of mTOR, MEK, and BRD4 (which targets c-MYC). Because the combined inhibition of mTOR with either MEK or BRD4 induced more pronounced tumor shrinkage in xenografts than inhibition with mTOR alone, the authors conclude that co-inhibition of mTOR with either MEK or c-MYC may prove efficacious in tumors harboring EIFIAX and RAS mutations.
Journal: Cancer Immunology Research
Research indicates that various components of the complement system, a group of proteins of the innate immune system, are enriched in the tumor microenvironment where they promote tumor progression; the role of complement C3 in suppressing antitumor immunity has yet to be fully elucidated. In this study, the authors found that intracellular activation of tumor-cell derived C3, which generates the cleavage product C3a, enables the binding of C3a to its protein receptor C3aR, activating PI3Kγ signaling in tumor-associated macrophages and promoting immunosuppressive activity. Additionally, the authors found that mice bearing tumors with high C3 expression were resistant to anti-PD-L1 treatment, while mice bearing tumors with low C3 expression had a more robust response to anti-PD-L1 therapy and longer survival. These results highlight tumor-derived C3 to be a potential target for immunotherapy and suggest that depleting C3 in tumor cells may be a viable strategy to promote antitumor immunity.
Journal: Clinical Cancer Research (February 15 issue)
Toll-like receptors (TLRs) initiate immune responses against bacterial, fungal, or viral pathogens; agonists of various TLRs are in clinical development as potential immunotherapy treatments for cancer. This first-in-human study evaluated the safety, efficacy, and immunologic activity of intratumoral G100, a synthetic TLR4 agonist, in 10 patients with Merkel cell carcinoma (MCC). Treatment was safe, with most adverse events being grade 1 or 2 injection-site reactions. Intratumoral administration of G100 resulted in inflammatory changes in the tumor microenvironment, such as increased infiltration of CD8+ and CD4+ T cells and the activation of chemokine- and cytokine-related genes, which corresponded with local tumor regression. All patients were alive at the time of follow-up analysis (median of 33.7 months), highlighting the potential efficacy of this immunotherapy treatment for patients with MCC.
Journal: Cancer Research (February 15 issue)
Mas Receptor Activation Slows Tumor Growth and Attenuates Muscle Wasting in Cancer
Cachexia, a condition characterized by the progressive loss of skeletal muscle, affects 40-80 percent of patients with advanced cancer and is estimated to account for 20-30 percent of all cancer-related deaths; the identification of effective treatment options for this syndrome is a critical unmet need. In this study, the authors investigated the activation of mitochondrial assembly receptor (MasR) for the treatment of cancer cachexia. They found that mouse models of cancer cachexia treated with the MasR agonist AVE 0991 slowed tumor growth, improved locomotor activity, diminished weight loss, and attenuated muscle wasting. These preclinical results identify AVE 0991 as a potential therapeutic for cancer patients with cachexia.
Journal: Molecular Cancer Therapeutics
MTORC1/2 Inhibition as a Therapeutic Strategy for PIK3CA Mutant Cancers
Mutations to the PIK3CA gene are relatively common in cancers, yet precision medicine strategies that target these aberrations have yet to be developed. Although mTOR inhibitors are often used off-label for patients with PIK3CA-mutant cancers, there is limited data to support this strategy. In this study, the authors found that patients with activating PIK3CA mutations in their tumors and treated with the MTORC1 inhibitor everolimus had minimal benefit, therefore they evaluated the efficacy of MTORC1/2 inhibition in several models of colorectal cancer (CRC) with PIK3CA and APC gene mutations. The researchers found that the MTORC1/2 inhibitor TAK-228 induced a treatment response in isogenic human cell lines, reduced murine MTORC1-resistant spheroids, and reduced colon tumors in mice. The authors conclude that MTORC1/2 inhibition warrants further clinical investigation in select patient populations with PIK3CA-mutant cancers.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Recent evidence indicates that physical activity is associated with better clinical outcomes among men with localized prostate cancer, however, the underlying mechanism for this correlation remains unknown. To determine if physical activity altered DNA methylation profiles in tumor tissues, the authors conducted a methylome-wide analysis in patients with localized prostate cancer who had self-reported physical activity and long-term follow-up data. They found that men who did vigorous physical activity at least once a week during the year before their diagnosis of prostate cancer were significantly less likely to develop metastatic-lethal disease; those who exercised vigorously at least weekly had a differentially methylated region in the promoter region of the CRACR2A gene, which reduced its expression. This research further confirms the association between physical activity and improved outcomes in localized prostate cancer and indicates that vigorous exercise may result in the epigenetic modifications of the CRACR2A gene.