AACR Annual Meeting 2019: Preclinical Steps Toward a Cancer Preventive Vaccine for Lynch Syndrome
Cancer prevention refers to measures that people can take to reduce their risk of developing cancer. These measures can include lifestyle changes, like eliminating tobacco use, maintaining a healthy weight, staying active, and limiting exposure of skin to ultraviolet light. As our scientific knowledge of cancer etiology and the biology of premaligancy has grown, so too has our ability to rationally develop targeted and immunotherapeutic interventions for cancer prevention, in particular for those who inherit genetic mutations that predispose them to developing cancer.
A preclinical study presented at the AACR Annual Meeting 2019 by Steven Lipkin MD, PhD, from Weill Cornell Medicine in New York, epitomizes this concept because the researchers report harnessing basic scientific discoveries about the biology of colorectal cancer in patients with Lynch syndrome to develop a vaccine that reduced intestinal tumors and improved survival in a mouse model of the disease.
What is Lynch syndrome?
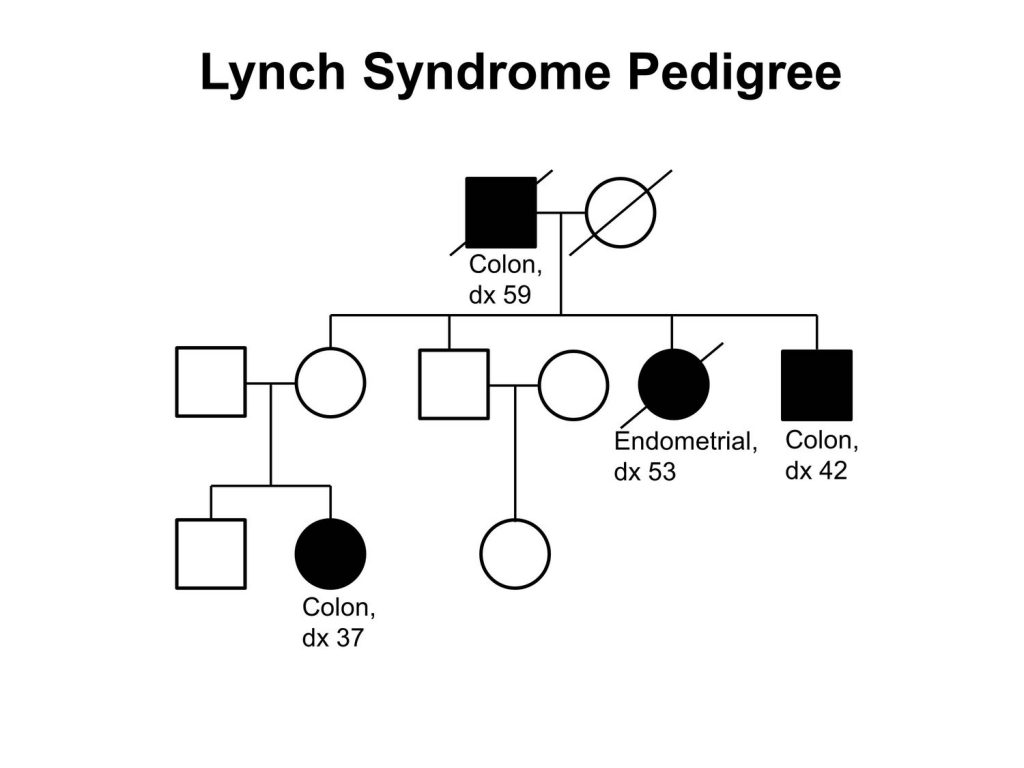
Lynch syndrome is an inherited disorder that increases a person’s risk of developing many types of cancer, including cancers of the bladder, colon, endometrium, gallbladder, liver, ovaries, rectum, stomach, skin, and small intestine. These cancers often arise at a younger age than would typically be expected. Because colorectal cancer is the most common cancer to arise in patients with Lynch syndrome the disease is sometimes referred to as hereditary nonpolyposis colorectal cancer.
Lynch syndrome affects about one in every 280 individuals in the United States, according to Lipkin. These individuals have a 70 to 80 percent lifetime risk of colorectal cancer he says. Overall, it is estimated that Lynch syndrome is the cause of between 3 percent and 5 percent of the cases of colorectal cancer diagnosed each year in the United States. This translates into up to 7,280 cases of Lynch syndrome–attributable cases of colorectal cancer in the United States in 2019, given that it is projected that there will be 145,600 new cases of the disease this year.
“Currently, frequent screening to detect precancers and early-stage cancer is the main approach used to prevent cancer in people with Lynch syndrome, although some people have risk-reducing surgeries and some take aspirin for colorectal cancer prevention,” said Lipkin in an AACR press release about the study he presented during the AACR Annual Meeting 2019.
What do we know about the biology of Lynch syndrome?
Mutations in five different genes—EPCAM, MLH1, MSH2, MSH6, and PMS2—can cause Lynch syndrome. Mutations in these genes cause defects in one of the many pathways that cells use to repair damaged DNA, the mismatch repair pathway. The mismatch repair deficiency that results from mutations in the five Lynch syndrome–associated genes leads to the gradual accumulation of other mutations in the genome, which can lead to uncontrolled cell growth and ultimately cancer.
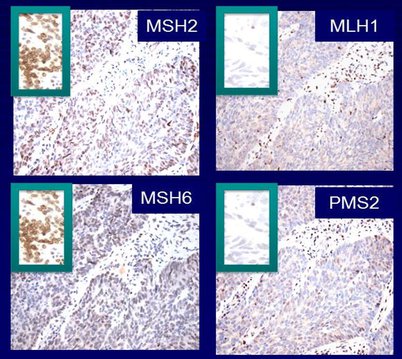
In patients with Lynch syndrome, the mutations predominantly accumulate in parts of the genome called microsatellites, which are regions of DNA in which a short sequence, typically one to nine nucleotides in length, is repeated over and over. As a result, these tumors are referred to as having high microsatellite instability (MSI-high). Most of the microsatellites affected in patients with Lynch syndrome are in noncoding regions of the genome, but some are found in coding regions. Interestingly, some of the affected genes are known to be cancer-related, for example, BAX and TGFbRII.
As discussed in a review article on the biology of MSI-high cancer, Lynch syndrome–associated coding microsatellite mutations can cause a shift in how the coding sequence of the gene is read, a so-called frameshift mutation. Frameshift mutations result in the generation of truncated or modified proteins. Modified proteins that contain novel tumor-specific peptides, which are also called neoantigens, can trigger an immune response directed at the tumor.
Importantly, in patients with Lynch syndrome, the number of genes with coding microsatellite mutations resulting in frameshift mutations and neoantigens is relatively well defined. Moreover, immune responses against these neoantigens have been detected in patients with Lynch syndrome.
How can we harness understanding of Lynch syndrome biology for cancer prevention?
The knowledge that coding microsatellite mutations generate neoantigens that can stimulate tumor-specific immune responses in patients with Lynch syndrome led to the idea of developing vaccines to preemptively trigger robust immune responses to prevent cancer from developing in Lynch syndrome patients.
In his presentation at the AACR Annual Meeting 2019, Lipkin reported promising results with a vaccine in a preclinical model of Lynch syndrome that suggest that it should be possible to develop a cancer preventive vaccine for patients with Lynch syndrome.
“We are extremely encouraged by our preclinical data, which suggest that rationally designed cancer vaccines may prevent some cancers associated with Lynch syndrome. We are in the process of designing a clinical trial to test this hypothesis,” said Lipkin in the AACR press release about the study.
Lipkin and his colleagues started by studying cancer prevention in a mouse model of Lynch syndrome. One of the first steps they took was to analyze intestinal tumors from Lynch syndrome mice for mutations in coding microsatellites. They identified 13 coding microsatellites that had at least one mutation that occurred in 15 percent or more of the tumors.
After determining which mutations were likely to generate neoantigens, the research team tested how effective the most promising of these neoantigens were as a vaccine.
They found that vaccination of Lynch syndrome mice with four of the neoantigens generated because of the coding microsatellite mutations induced robust neoantigen-specific immune responses. It also significantly reduced intestinal tumor burden and improved survival compared with nonvaccinated Lynch syndrome mice. In nonvaccinated Lynch syndrome mice, the median intestinal tumor burden was 61 mg and overall survival was 241 days, compared with 31 mg and 380 days, respectively, in vaccinated Lynch syndrome mice.
Given that some patients with Lynch syndrome take aspirin for colorectal cancer prevention, the researchers investigated whether combining vaccination with administration of aspirin or the nonsteroidal anti-inflammatory drug naproxen could improve overall survival for the Lynch syndrome mice. They found that only the vaccine–naproxen combination significantly improved overall survival for the mice compared with vaccination alone; in mice receiving the naproxen combination treatment, overall survival was 541 days versus 380 days in mice receiving only the vaccine.
“Our preclinical data are very exciting because they provide strong support for continuing the investigation and development of immunoprevention strategies for patients with Lynch syndrome,” said Lipkin. Fierce Biotech and The Daily Mail covered Lipkin’s study.
The data from this study highlight how breakthroughs in our understanding of the genomic and immunological events occurring during cancer initiation can transform cancer prevention research, as world-renowned leaders in the field predicted would happen in a Special Report published in the AACR journal Cancer Prevention Research in January 2016. We look forward to showcasing more of these cutting-edge advances on Cancer Research Catalyst in the future.




