AACR Annual Meeting 2019: Promising Data from CAR T-cell Clinical Trials for Advanced Solid Tumors
A pair of studies presented at the AACR Annual Meeting 2019 demonstrated encouraging clinical outcomes with two different chimeric antigen receptor (CAR) T-cell therapies for patients with advanced solid tumors.
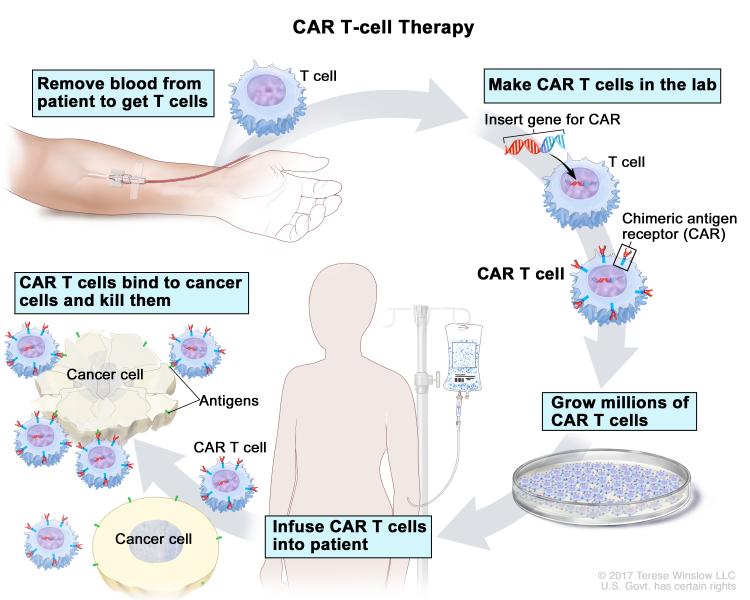
CAR T-cell therapy is a type of immunotherapy in which T cells are removed from a patient’s body and genetically modified so that they can recognize the patient’s cancer cells. The modified T cells, when reintroduced into the patient’s body, multiply and attack cancer cells. The U.S. Food and Drug Administration has approved two CAR T-cell therapies for blood cancers so far: tisagenlecleucel (Kymriah) for treating certain patients with acute lymphoblastic leukemia or non-Hodgkin lymphoma (NHL), and axicabtagene ciloleucel (Yescarta) for treating certain adults with NHL.
Given the success of CAR T-cell therapies for blood cancers, the most anticipated next step in the field is the development of this type of immunotherapy for solid tumors. Developing CAR T-cell therapies for solid tumors, however, has been challenging, and researchers in the field have had several failed attempts. Challenges include finding the right target to direct the T cells, the ability to expand in an often-immunosuppressive tumor microenvironment, yielding an effective antitumor response, and avoiding adverse side effects. This recent review article summarizes the challenges with developing CAR T-cell therapy for solid tumors and strategies to overcome the hurdles, and lists some of the ongoing clinical trials in which CAR T cells that target tumor antigens, including GD2, CEA, and EGFR, are being currently tested against a variety of solid tumors.
Data presented here at the conference have demonstrated that it is possible to develop CAR T-cell therapies for solid tumors that are not only safe but also yield clinical benefit for patients.
Mesothelin-targeted CAR T-cell therapy for patients with malignant pleural disease
In a phase I clinical trial, a team of researchers from Memorial Sloan Kettering Cancer Center tested whether mesothelin-directed CAR T cells that they developed would be safe and effective in patients with malignant pleural disease from mesothelioma and lung and breast cancer.
The CAR T cells the team developed, called IcasM28z, target the cell-surface protein, mesothelin, which is expressed on the majority of cancer cells. The team also worked to deliver the CAR T cells directly to the tumor site using regional delivery techniques. “If this approach is successful, 2 million patients with mesothelin-expressing solid tumors per year in the United States will be eligible for this treatment,” said Prasad S. Adusumilli, MD, who presented the study. This article in Cancer Discovery reviews the types of solid tumors that express mesothelin, and the potential of mesothelin-directed CAR T-cell therapy to treat such malignancies.
Because some normal cells in the body express very low levels of mesothelin, it is possible that the mesothelin-targeted CAR T cells could be toxic to them. The researchers, including the study’s senior author Michel Sadelain, MD, PhD, therefore incorporated an Icaspase-9 (inducible caspase-9) safety “suicide” switch that can be activated in a patient’s body in case of an unexpected toxicity, to eliminate all CAR T cells. However, the investigators have not observed any major toxicity with the doses tested in this trial.
Using an interventional radiology procedure, the investigators injected IcasM28z CAR T cells directly into the pleural cavity in 21 patients with malignant pleural disease (19 with malignant pleural mesothelioma, one with metastatic lung cancer, and one with metastatic breast cancer).
During the 38-week evaluation, the IcasM28z CAR T cells were found to be persistent in the peripheral blood of 13 patients, which was associated with more than 50 percent reduction in the levels of serum mesothelin-related protein and evidence of tumor regression on imaging studies.
One patient with mesothelioma underwent curative-intent surgery followed by radiation therapy to the chest. In preclinical studies, the researchers found that in large tumors, the CAR T cells became functionally exhausted even while residing in the tumor. Treatment with anti-PD-1 agents could reactivate the exhausted CAR T cells and eradicate the tumors in a proportion of mice. Based on this rationale, 14 patients went on to receive anti-PD1 checkpoint blockade agents. After up to 21 cycles of treatment with an anti-PD1 agent, two patients had complete metabolic response on PET scans at 60 and 32 weeks, respectively, and these responses are ongoing at the time of this reporting; five patients had partial response; and four had stable disease.
“Combining rationally developed strategies—such as interventional radiology, T-cell genetic engineering, and newer immunotherapy agents—has produced encouraging results and provides rationale to further investigate this approach in aggressive, therapy-resistant cancers such as mesothelioma, for which the currently available treatment options are not optimal,” Adusumilli noted in a press release.
HER2-targeted CAR T-cell therapy for patients with advanced sarcoma
Current treatment options for children and adults with recurrent or treatment-resistant sarcomas are limited. Curative salvage chemotherapy regimens are available for some types of sarcoma, but the chance of success is low, and the therapies can be quite toxic. In a phase I clinical trial, Shoba Navai, MD, from the Center for Cell and Gene Therapy at Baylor College of Medicine, Texas Children’s Hospital, and Houston Methodist Hospital studied the safety of administering HER2-targeted CAR T cells in combination with lymphodepletion chemotherapy in patients with advanced HER2-positive sarcomas.
The exact percentage of sarcomas that express HER2 on the tumor surface is not known, but osteosarcoma, one of the most common types of sarcoma, has been reported to be HER2-positive in up to 40 percent of patients, and HER2-positivity is associated with a higher likelihood of tumor metastasis, Navai said. However, HER2-targeted antibodies, such as trastuzumab, have not been effective for these patients in clinical studies.
“Our group has previously shown in the laboratory that HER2-directed CAR T cells are better at targeting low levels of HER2 on tumor cells compared with trastuzumab, so these CAR T cells may have antitumor activity in patients with sarcoma even when HER2-antibody therapies do not,” Navai said.
Navai and colleagues, including study lead investigator Meenakshi Hegde, MD, treated 10 patients with up to three infusions of HER2-targeted CAR T cells after lymphodepletion with either fludarabine or fludarabine and cyclophosphamide. The patients were 4 to 54 years old, had refractory/metastatic HER2-positive sarcoma (five with osteosarcoma, three with rhabdomyosarcoma, and one each with Ewing sarcoma and synovial sarcoma), and had received up to five prior salvage therapies.
Patients who responded to the initial HER2-directed CAR T cell treatment received up to an additional five infusions of CAR T cells without lymphodepletion. Expansion of the CAR T cells was observed in all but two patients with a median peak expansion on day 7, and CAR T cells could be detected in all patients six weeks after infusion.
A pediatric patient with osteosarcoma that had metastasized to the lungs has an ongoing complete response for 32 months. Three patients had stable disease, and five had progressive disease. Another pediatric patient whose rhabdomyosarcoma had metastasized to the bone marrow had a complete response for 12 months but relapsed later; retreatment with CAR T cells resulted in a complete response that has been ongoing for 17 months. Proteomic analysis of the patient’s serum at multiple time points demonstrated antibody responses against several intracellular proteins involved in the cell cycle, cell growth, and cell signaling as well as in tumor processes such as invasion and metastasis.
“Some antibodies in this patient’s blood were newly detected and maintained after CAR T-cell infusions, suggesting that in addition to the expected direct effect of the CAR T cells targeting HER2 on the tumor, they may have engaged the patient’s own immune system,” Navai noted.
Overall, the patients in this trial had limited treatment-related toxicities. Most patients developed fever within 24 hours of the CAR T-cell infusion, which resolved with supportive care within three days of onset, according to Navai. All patients had expected decreases in their blood counts following chemotherapy that subsequently improved, and none developed infections secondary to low blood counts. None of the patients experienced decreased heart function or pulmonary toxicities.
“Our study shows that HER2-targeted CAR T-cell therapy given in combination with lymphodepletion chemotherapy is safe, and though very early, it shows promising antitumor activity in some patients with advanced HER2-positive sarcomas,” Navai said.
We look forward to following up on these exciting cancer research advancements and keeping you updated on Cancer Research Catalyst.