Taking Aim at Tobacco: Highlights From Annual Meeting Symposium
Editor’s note: At the 2019 AACR Annual Meeting in Atlanta, the AACR’s Tobacco Products and Cancer Subcommittee hosted a symposium session, moderated by subcommittee chair Roy Herbst, MD, PhD. The session convened experts from the U.S. government (Mitchell Zeller, JD, director of the FDA Center for Tobacco Products, and Brian King, PhD, MPH, deputy director of Research Translation at the CDC), a cancer prevention expert from the United Kingdom (Alison Cox, PhD, director for Cancer Prevention, Cancer Research UK), and a local youth tobacco prevention specialist (D’Jillisser Kelly from Learn to Grow, Atlanta). The session focused on the growing problem of youth electronic cigarette (e-cigarette) use in the United States. Here, Herbst and Zeller offer their thoughts on issues covered in this session.
Roy Herbst on the e-cigarette crisis and how the AACR is fighting it
The tobacco product landscape is evolving and the AACR Tobacco Products and Cancer Subcommittee has been cognizant of the shift from combustible products (e.g., cigarettes) to alternative nicotine delivery systems, such as e-cigarettes. While e-cigarettes may be a less risky alternative than smoking combustible products, these products still present risks and the AACR has been active in trying to keep children from having access to them. By submitting comment letters to the FDA, hosting briefings on Capitol Hill, and working with members of Congress on various pieces of legislation, the AACR has remained committed to preventing another generation of youth from becoming addicted to dangerous tobacco products. On Wednesday, June 12, the AACR held a congressional briefing where experts further discussed the surge in e-cigarette use among middle and high school students.
E-cigarettes appeared on the U.S. market in 2007 and have evolved from early disposable devices to the currently popular rechargeable pod-based devices (such as Juul). Youth use of these products has soared and, in 2018, 20 percent of high school students were using e-cigarettes. Additionally, high school students’ use of cigarettes had been steadily declining for the past 20 years but, within the past couple of years, the decline in use has stagnated, leading public health officials to question whether e-cigarette use is acting as a gateway to conventional cigarette use. As Brian King stated during the Annual Meeting session, “What we don’t want to be doing, particularly when it comes to kids, is playing a game of public health Whack-A-Mole where we allow certain products to go down and others to go up as we’re very concerned about youth use of all forms of tobacco products.”
Although e-cigarettes may play an important role in helping current adult smokers transition to a less harmful nicotine-containing product, and certain types of e-cigarettes used in a certain frequency may help adult smokers quit, the safety and relative effectiveness of e-cigarettes versus one of the seven FDA-approved cessation products is currently unknown. Juul was developed to be incredibly efficient in the delivery of nicotine and, while this may be beneficial in helping current smokers quit the use of cigarettes, it can be harmful to the developing adolescent brain.
Interestingly, although e-cigarettes are on the market in the United Kingdom (UK), that country has not experienced the same public health crisis of youth use that the U.S. has. Alison Cox shared that, in the UK, e-cigarettes are the most popular smoking cessation aid, but e-cigarette use by never-smokers remains negligible. Although British youth may experiment with these products, regular use in the youth population remains low. Cox attributed these differences to the fact that the UK heavily restricts the marketing of these products and limits the nicotine concentration of e-cigarettes to 20 mg/ml. By contrast, the nicotine concentration of a Juul pod is the U.S. is around 60 mg/ml.
In December 2018, U.S. Surgeon General Jerome Adams, MD, MPH, issued an advisory on youth e-cigarette use, saying that parents, teachers, health professionals, and government officials must take “aggressive steps” to keep children from using e-cigarettes. The importance of community engagement and educational campaigns was highlighted in the advisory, so groups such as D’Jillisser Kelly’s Learn to Grow organization are essential to help educate children, parents, and teachers on what these devices look like and the dangers of nicotine addiction, and to provide resources on how to quit if these products are already being used.
The FDA has a plan in place, called the Youth Tobacco Prevention Plan (YTPP), to address the surge in youth use of e-cigarettes, including various efforts to inform teens, their families, and educators about e-cigarettes and their associated health risks, and tobacco retailers about the importance of verifying age before selling any tobacco product. Mitch Zeller shared the details of this plan during the session and provides a summary below.
Mitch Zeller on the FDA’s Youth Tobacco Prevention Plan: Because no minor should be using any tobacco product
Protecting our nation’s youth from the dangers of tobacco products is one of the agency’s most important responsibilities. No child should be using any tobacco product, and the agency’s Youth Tobacco Prevention Plan—a part of the agency’s “Comprehensive Plan for Tobacco and Nicotine Regulation” announced as a regulatory roadmap in July 2017—focuses on stopping a new generation of kids from becoming hooked on tobacco products, especially e-cigarettes.
The YTPP relies on three main strategies to protect youth from the toll of tobacco: 1) preventing youth access to tobacco products; 2) curbing the marketing of these products to youth; and 3) educating youth and their families about tobacco products’ dangers, while educating retailers about their key role in protecting youth.
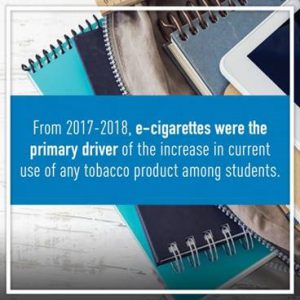
Effectively addressing youth tobacco access and use is crucial, and is especially pressing given that youth use of e-cigarettes has surged recently. In alarming findings from the National Youth Tobacco Survey (NYTS), released in 2018 by the FDA and the Centers for Disease Control and Prevention, there was a 78 percent increase between 2017 and 2018 in current e-cigarette use among high school students and a 48 percent increase among middle school students. The total number of middle and high school students currently using e-cigarettes rose to 3.6 million in the 2018 survey, which is 1.5 million more than in the previous year. NYTS authors suggested that the rise in e-cigarette use between 2017 and 2018 was due to the recent popularity of certain types of “pod-based” e-cigarette products such as Juul. Youth e-cigarette users also used them more frequently and more youth used flavored products in 2018 than in the previous year.
The FDA’s forceful response
The FDA is taking ongoing forceful action under the YTPP to stem the youth e-cigarette epidemic. Some actions have been designed specifically to address concerns about the pod-based products, whose characteristics—such as high levels of nicotine and a small, discreet design and hard-to-see emissions—may be factors in youth use.
For example, in the summer of 2018, under the YTPP, the FDA conducted a nationwide undercover blitz to crack down on the sale of e-cigarettes to minors by both brick-and-mortar and online retailers. Based on this largest coordinated enforcement effort in the FDA’s history, the agency issued more than 1,300 warning letters and civil money penalty complaints to retailers found to be selling Juul and other e-cigarette products to minors.
In additional efforts, the FDA worked to preclude online sales of e-cigarettes to youth by preventing online listings; sent regulatory letters to Juul and others requiring submission of documents to support a better understanding of issues related to youth use of their products; and requested plans from the makers of the most popular e-cigarettes describing how they will address the widespread youth access to, and use of, their products.
In another example of its actions under the YTPP, the FDA warned several companies to stop selling e-liquids whose labeling and/or advertising caused them to resemble kid-friendly food products such as juice boxes, candy, or cookies. The misleading imagery presents the risk of serious harm to kids who could confuse these e-liquid products for something safe to eat or drink.
Focus on flavors
This past March, the FDA issued a draft guidance that, among other items, outlined policy changes and enforcement priorities the agency may consider with respect to certain flavored tobacco products. The changes focused specifically on reducing the youth appeal of, and access to, certain flavored e-cigarettes and cigars. The public comment period on this draft guidance recently ended, and the FDA is currently reviewing comments with the intent of issuing a final guidance.
More about current initiatives
Alongside these compliance and enforcement efforts, the agency is continually advancing a broad range of additional science-based regulatory and educational efforts that include developing regulations; reviewing tobacco product applications; overseeing pivotal research; and preventing youth initiation and encouraging adult cessation through public education campaigns designed to promote behavior change.







Cancer is a major problem that is difficult to diagnose. We all try to diagnose it. Your blog is very good. Ever got information about it.