Ovarian Cancer: Examining the Microenvironment and Mutational Landscapes to Tailor Treatments
The AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, held this year at the Hynes Convention Center in Boston Oct. 26-30, has once again brought together members of academia, pharmaceutical industry, federal regulatory agencies, and all other stakeholders in the cancer drug development space from across the globe to discuss the most up-to-date advances.
A couple of studies presented at the conference set out to address an important question in cancer research – why is ovarian cancer so hard to treat?
In a Spotlight session on Monday, “Understanding and Modeling the Response to Immunomodulation,” researchers presented new data utilizing innovative precision medicine approaches to understand how mutational drivers associated with DNA repair proteins influence tumor-immune evasion and define the ovarian cancer microenvironment.
Ovarian cancers account for 1.3 percent of all cancers diagnosed in the United States, however, the five-year survival rate for this disease in advanced stages is just 29 percent. High-grade serous ovarian cancer (HGSOC), which originates in the epithelium of the fallopian tube, is the most common and most aggressive histologic subtype of ovarian cancer and accounts for about 80 percent of mortalities.
HGSOC is often responsive to platinum-based chemotherapy given after cytoreductive debulking surgery. One of the main reasons HGSOC responds to platinum-induced DNA damage is that these cancers are often chromosomally unstable due to impaired DNA repair mechanisms. About 20 percent of HGSOC cases are associated with mutations in the genes BRCA1 or BRCA2, which play a role in the DNA repair pathway called homologous recombination (HR).
Poly ADP ribose polymerase (PARP) inhibitors, a class of cancer therapeutics, inhibit PARP, one of the DNA damage repair proteins that the cancer cells use to fix their DNA, thus forcing the cells to die. The U.S. Food and Drug Administration (FDA) has approved three PARP inhibitors so far: olaparib (Lynparza) and rucaparib (Rubraca) to treat certain ovarian cancers with BRCA gene mutations, and niraparib (Zejula) to treat both BRCA mutation-positive and BRCA mutation-negative ovarian cancers, and for HR deficiency-positive ovarian cancers. While these developments dramatically increased treatment options and response rates for patients with ovarian cancer, most patients eventually develop resistance and face disease progression.
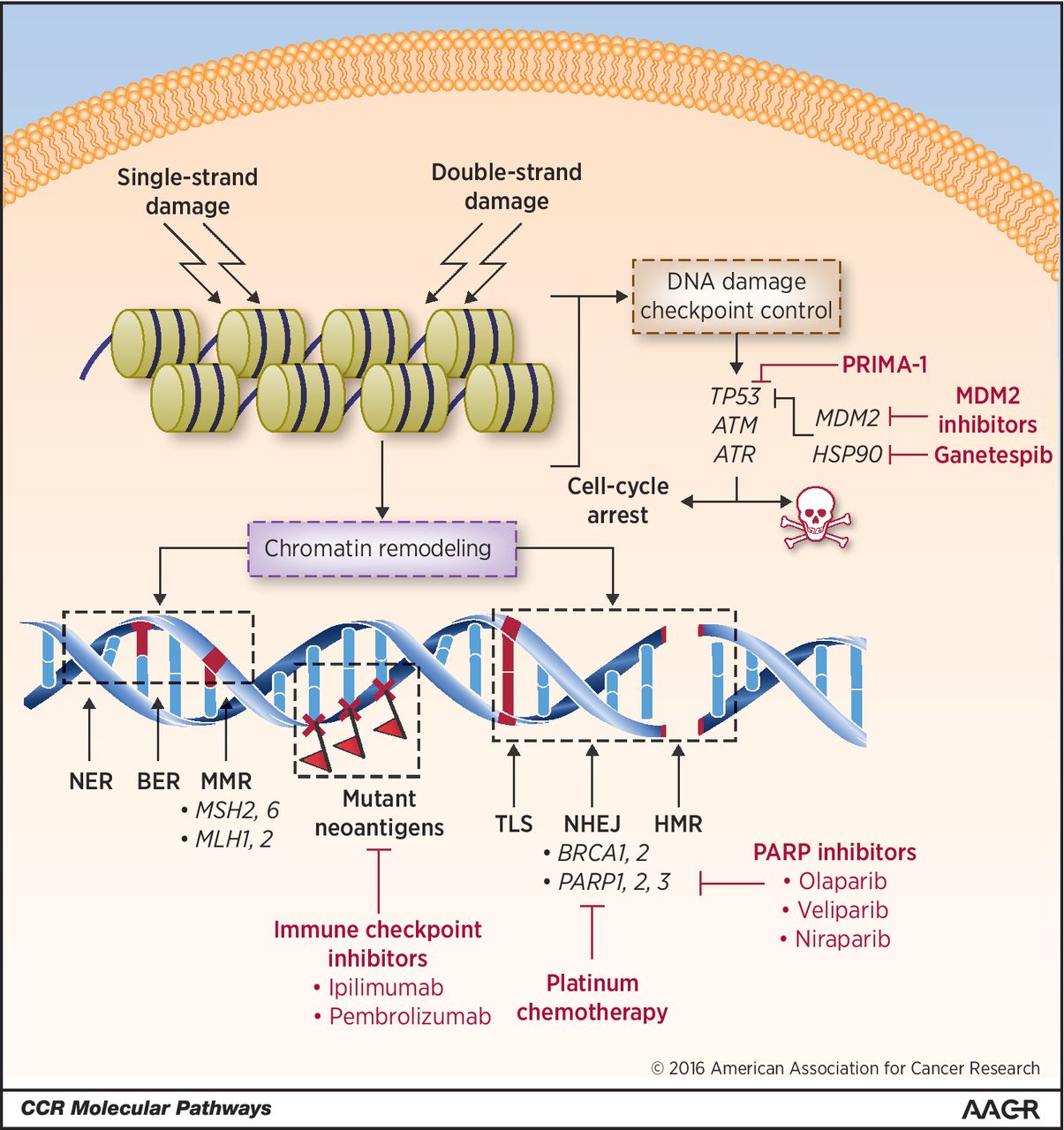
Cancers with DNA repair defects tend to have increased mutational load and are likely to be susceptible to immunotherapeutic targeting. In May 2017, the FDA approved the immune checkpoint inhibitor pembrolizumab (Keytruda) to treat any type of solid tumor that has been identified as having one of two biomarkers, microsatellite instability–high or mismatch repair–deficient. However, most ovarian cancers, despite carrying DNA repair defects, do not respond to this type of immunotherapy. So far, it is not clear why.
Uniquely designed ovarian cancer models help study the interplay between DNA damage repair capabilities and immune evasion
In the Spotlight session, two presenters discussed using new ovarian cancer models to understand the complex relationship between the types of DNA damage repair deficiencies and immune evasion. Preclinical animal models and cell lines currently used to study the sensitivity and responses of ovarian cancers to different treatments have limitations, noted Sonia Iyer, PhD, a postdoctoral fellow in the laboratory of Robert Weinberg, PhD, at the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, who presented the first study in the Spotlight session.
Iyer met Shuang Zhang, PhD, from the laboratory of Benjamin Neel, MD, PhD, at NYU Langone Health at an AACR conference a couple of years ago. Their collaboration resulted in the development of two-dimensional (2D) cell lines and three-dimensional (3D) organoids of ovarian cancers to study how the combination of genetic alterations and DNA repair proficiency of ovarian cancer cells dictate the immune composition of the tumors.
In her presentation, Iyer noted that the 2D ovarian cell line models she developed were designed to capture how common driver mutations, such as TP53, BRCA1, PTEN, MYC, Cyclin E1 (CCNE1), AKT2, and KRAS, shape the interactions between cancer cells and components of the microenvironment, including immune cells. Data showed that HR-deficient and HR-proficient HGSOC tumors have distinct tumor microenvironments, and that combining an immune checkpoint inhibitor with an inhibitor of the DNA damage response protein, checkpoint kinase 1 (Chk1), produced antitumor effects and promoted cytotoxic T-cell infiltration in 2D cell lines with genes associated with HR-proficiency.
In the second presentation, Zhang discussed the development of the 3D organoids. In a paper published in May in Nature Medicine, the researchers established a protocol to develop organoid lines from 32 patients with different subtypes of ovarian cancer. The authors note that unlike traditional cell lines, tumor organoid cultures are 3D in vitro systems that reflect the characteristics of the tumor from which they are derived. Because of the way they are established and maintained, they are best suited to study tumor heterogeneity and drug sensitivity, and to predict patient response.
Zhang noted that because the organoids contained combinations of several driver mutations seen in human HGSOC, they differed in several important characteristics of tumor development, including proliferation, differentiation, tumorigenic capacity, and behavior, when they were injected into syngeneic mice. For instance, HGSOC organoids with BRCA1 mutations and CCNE1 overexpression induced an immune microenvironment that had increased T-cell infiltration and myeloid-derived suppressor cells (MDSCs), while the microenvironment induced by organoids with PTEN mutations had lower levels of T-cell infiltration but increased MDSCs and macrophages.
The studies showed that CCNE1-overexpressing organoids were sensitive to treatment with gemcitabine, leading to decreased tumor burden. Combining gemcitabine with anti-CTLA4 and anti-PDL1 immune checkpoint inhibitors yielded complete responses in mice with extensive metastatic disease. The presentations highlight the potential of designing genotype-informed combination therapies to identify new treatments and better outcomes for patients with ovarian cancer.
The impact of immune microenvironment on antitumor responses
Earlier at the conference, on Saturday, George Coukos, MD, PhD, from University of Lausanne, Switzerland, delivered a keynote lecture titled “Dissecting antitumor response in ovarian cancer.”
In his talk, Coukos detailed the role of tumor infiltrating lymphocytes (TILs) in influencing immune responses and survival in patients with ovarian cancer. His research showed TILs are activated in ovarian cancers and are specific to the tumor neoantigens, which suggests that these cancers may be amenable to TIL therapy and immune mobilization using vaccines of whole tumor lysates.
Coukos noted that the antigen-activated TILs increased the expression of the checkpoint proteins CTLA4 and PD1, suggesting a combination of inhibitors of these two checkpoints could promote antitumor effects in ovarian cancer.
Data also revealed proangiogenic vasculature acts as a barrier to T-cell engraftment in the tumor. In a paper published in Science Translational Medicine in 2018, Coukos and colleagues demonstrated that a combination of personalized vaccine generated by autologous dendritic cells pulsed with oxidized autologous whole-tumor cell lysate, the angiogenesis inhibitor bevacizumab, and low-dose cyclophosphamide was safe and effective in eliciting antitumor immunity in patients with recurrent ovarian cancer.
Further studies also showed that the chemokine CCL5, expressed by ovarian cancer cells, is required for T-cell engraftment into the tumor, and that the cooperation between tumor-derived CCL5 and IFN-γ-inducible CXCR3 ligands secreted by myeloid cells plays a role in orchestrating T-cell infiltration in immune-responsive tumors. These data were published in Cancer Cell.
In a paper published in the Journal of Clinical Oncology in September titled “Immunotherapy in ovarian cancer: Are we there yet?” Coukos and colleagues review immunotherapeutic approaches currently being tested for ovarian cancers and share insights into future development in this area.