AACR Virtual Annual Meeting I: Two Liquid Biopsy Tests Offer Promise of Early Detection of Cancer
Early detection of most cancers is an elusive and daunting task; nevertheless, it has remained a top priority for the cancer research community. Detecting cancer early has manifold advantages: It can increase a patient’s chances of successful treatment, prolong their survival, and substantially improve their quality of life.
Developing a blood-based test that is sensitive and specific enough to ensure an accurate diagnosis of cancer before it manifests clinically has many challenges. Liquid biopsy-based tests often utilize analysis of circulating tumor DNA (ctDNA), DNA from cancer cells circulating in the blood, to look for the presence of DNA alterations in the cancer cells. However, some cancers may not shed ctDNA into the blood at levels above the detection limit of a liquid biopsy-based test. Normal processes of the components of the blood, such as clonal hematopoiesis, could generate mutations and potentially confound the test results. DNA fragments from cancers of the central nervous system may not cross the blood-brain barrier to enter the blood stream. These are just a few examples of the myriad hindrances in developing a liquid biopsy-based test to detect cancer early. A false-negative test result could prove to be a fatal mistake for a patient, and a false-positive test result could be devastating to a patient, besides leading to an array of unnecessary additional tests.
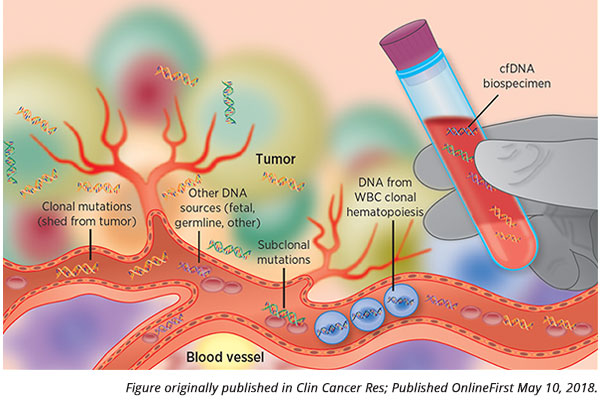
A pair of studies presented at the AACR Virtual Annual Meeting I in the Clinical Plenary Session titled “Early Detection of Cancer and ctDNA” showcased two liquid biopsy-based tests that could potentially distinguish blood samples from patients who had cancer from blood samples from patients who did not have cancer and could identify the tissue in which the cancer originated.

The existence of cell-free DNA in the blood was first discovered and described as early as 1948, and its implication in cancer was first described in 1977, explained Session Chair Alberto Bardelli, MD, associate professor in the Department of Oncology at University of Turin, Italy. “However, only recently, thanks to the advancements of next-generation sequencing and other molecular diagnostic tools, it became possible to analyze ctDNA in high details and with specificity and sensitivity, using liquid biopsy.”
Diagnosing cancer in individuals with suspicion of the disease
David D. Thiel, MD, a physician in the Department of Urology at Mayo Clinic, Florida, presented data on a multicancer detection test that he developed along with scientists from the Circulating Cell-free Genome Atlas (CCGA) Consortium and from GRAIL Inc.
“Many cancers are detected too late,” began Thiel, who noted that detecting cancer prior to metastatic spread could increase survival by 10-fold, and that existing paradigms to screen for the cancer types for which tests are available currently are suboptimal. “A simple and noninvasive multicancer early detection test could potentially decrease cancer-related mortality,” he noted. Thiel went on to describe the development of such a test that is being validated in a population-scale clinical study.

Thiel and colleagues developed the multicancer detection test through a prospective, longitudinal, case-control study of samples collected from 15,254 individuals from 142 sites in North America, of whom 8,584 had cancer and 6,670 did not. This test is intended to be used along with guideline-recommended screening and not replace it, he noted.
The test utilizes a whole-genome bisulfite sequencing approach that relies on the methylation signature of cancer cells. This methylation-based test was validated, and the data were published recently in Annals of Oncology. Data from this second case-control sub-study of about 5,000 samples showed that in the validation phase, the test had 99.3 percent specificity and 67.3 percent sensitivity in a prespecified set of 12 cancer types of stages 1 to 3. The test could predict the tissue of origin in 96 percent of the samples and it was accurate in 93 percent of them.
In his AACR Virtual Annual Meeting I presentation, Thiel reported on the assessment of the utility of the test in a subset of 303 individuals with a high clinical suspicion (HCS) of cancer through clinical and/or radiological assessments but without a confirmed pathology-based diagnosis at the time of enrollment. Subsequent pathological analysis showed that in the training set of 213 subjects, 164 had clinically confirmed cancer; in the validation set of 90 subjects, 75 of them had clinically confirmed cancer.
In the HCS subgroup, the specificity of the multicancer detection test, or the ability of the test to accurately detect the absence of cancer in individuals clinically confirmed as not having cancer, was 100 percent in both the training and validation sets. “The high specificity, although with a small sample size, suggests that the false-positive rate was not elevated in individuals with high risk, i.e., high clinical suspicion of cancer, versus the enrolled population,” Thiel said.
The sensitivity of the test, or the ability of the test to detect any cancer of stages 1 to 4 in clinically confirmed cancer patients, was 40.2 percent and 46.7 percent in the training and validation sets, respectively. Excluding samples of kidney cancer, which has a low tumor fraction in the blood, improved the sensitivity of the test.
The test could predict the tissue of origin in 93.9 percent and 100 percent of the cases in the training and validation sets, respectively. This prediction was accurate in 85.5 percent and 97.1 percent in the training and validation sets, respectively. “This targeted methylation-based multicancer early detection test could potentially be utilized to accelerate the diagnostic workup of individuals with high suspicion of cancer,” Thiel said.
Diagnosing cancer in women with no history of the disease
“The ability to identify cancer through blood testing is one of the most exciting advances in cancer diagnostics,” began Nickolas Papadopoulos, PhD, professor of oncology and pathology at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center in Baltimore.

Papadopoulos described the development of the multicancer detection test, DETECT-A, and its testing in a prospective interventional study of 10,000 women, ages 65-75, with no prior diagnosis of cancer, recruited through Geisinger Health System. Participants were screened for cancer using the DETECT-A test, which is designed to analyze a panel of 1,933 bases in the DNA covering regions of 16 genes and nine proteins implicated in cancer.
After accounting for any confounding effects from clonal hematopoiesis, patients whose samples had positive test results were followed up with corresponding imaging studies using positron emission tomography–computed tomography (PET-CT) when applicable and followed up further.
Data showed that the DETECT-A blood test could prospectively detect cancer in individuals not previously diagnosed with the disease through other methods. The test could detect 10 cancer types, which is seven more than the three cancers for which there are standard screening methods – breast, lung, and colorectal cancer, Papadopoulos noted. Of the positive cases, 65 percent were localized or regional disease. The positive-predictive value of the test was 19 percent, which rose to 41 percent when the results were combined with the results from PET CT.
Of the abnormalities detected, 57 percent were mutations, and the rest were alterations in the proteins.
Papadopoulos noted that the DETECT-A test could potentially prompt an early intervention. He added that it should be possible to incorporate the blood test into routine clinical care without disrupting the standard-of-care screening process for cancers when they are available, and, in fact, it could complement the standard screening tests rather than replace them. The test doubled the number of cancers detected by standard screening methods.
The blood test could potentially be integrated into the clinic without requiring invasive, wasteful follow-up tests, he said. About 1 percent of the study participants with a false-positive test result had to undergo further confirmatory procedures.
“It is feasible for a minimally invasive blood test to safely detect several types of cancers in individuals without known cancer, leading to treatment with intent to cure in a subset of them,” Papadopoulos said. This study could inform the design of a randomized trial to further study the clinical utility, cost-effectiveness, and benefit-to-risk ratio of future tests, he concluded. The study was covered by The Associated Press and published in numerous media outlets.
How will these tests be used?

Discussant of the session, David G. Huntsman, MD, professor in the Departments of Pathology and Laboratory Medicine and Obstetrics and Gynecology at the University of British Columbia, raised some important questions about the sensitivity of the tests and the duration of follow-up required to make meaningful assessments on the impact of these tests and the prospective studies, including on survival. He pondered whether multiple tests over time could provide more information.
“Ultimately, we would need to show improved survival measured by prospective studies, and both groups are moving in this direction, which is exciting and courageous,” Huntsman said. “The cost-effectiveness of any assay is going to be critical as we move forward, because no system has infinite resources.”



