AACR Virtual Annual Meeting I: Showcasing Technological Advances in Adoptive Cell Transfer
“The field of cell therapy is one of the most exciting fields in translational medicine,” began Katayoun Rezvani, MD, PhD, chair of the Adoptive Cell Transfer Therapy session at the AACR Virtual Annual Meeting I.
Rezvani, who is a professor of Stem Cell Transplantation and Cellular Therapy at The University of Texas MD Anderson Cancer Center, added, “It stands at the intersection of a variety of rapidly developing scientific fields, including immunology, cell engineering, gene editing, and clinical research.”

Adoptive cell transfer therapy is a type of immunotherapy in which immune cells are isolated from the blood of a patient with cancer, trained to target certain molecules present on the cancer cell, expanded in number, and reintroduced into the patient’s blood.
Chimeric antigen receptor (CAR) T-cell therapy, one of the groundbreaking adoptive cell transfer approaches, involves genetically modifying T cells removed from a patients’ body to express a CAR that recognizes a protein on cancer cells, and returning the cells to the patient’s blood circulation. The U.S. Food and Drug Administration (FDA) has approved two CAR T-cell therapies thus far to treat children and adults with certain types of acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL). Both these treatments target the protein CD19, expressed in many B-cell malignancies.
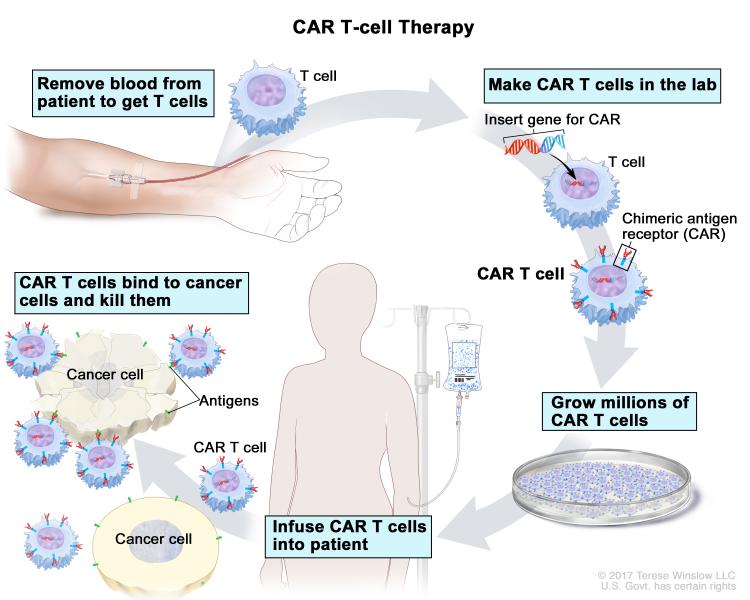
Previous blog posts have chronicled the FDA approvals of the two CAR T-cell therapies tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta); the exciting progress with improving the effectiveness of CAR T-cell therapies; developing a universal adaptor to expand their applicability; addressing resistance to this type of treatment; testing the applicability of pluripotent stem cells to develop off-the-shelf, universal CAR T cells; tinkering with CAR T cells to potentially treat solid tumors; and clinical trial data testing CAR T-cell therapies for solid tumors.
Tumor-infiltrating lymphocyte (TIL) therapy is another exciting approach to adoptive cell transfer that predates CAR T-cell therapies. This approach involves harvesting tumor-specific T cells from the tumor, expanding them, and infusing them back into the patient. TIL therapy pioneer Steven Rosenberg, MD, PhD, FAACR, chief of surgery at the National Cancer Institute, explained in this post how his new approach could potentially be used to treat ovarian cancer and other solid tumors. The AACR will present Rosenberg with the 2020 AACR-CRI Lloyd J. Old Award in Cancer Immunology.
Presentations at the AACR Virtual Annual Meeting I, in the Adoptive Cell Transfer Therapy session, featured the latest clinical developments in CAR T-cell therapies, TIL therapy, the utility of state-of-the-art technologies to accelerate the process of adoptive cell transfer therapy development, and a potential combination treatment for patients with lymphoma.
Dual antigen-targeting CAR T cells to reduce the risk of relapse
Haneen Shalabi, DO, assistant research physician in the Pediatric Oncology Branch at the National Cancer Institute Center for Cancer Research, discussed the clinical testing of a bivalent, bispecific CAR T-cell therapy in children and young adults with recurrent or refractory B-cell malignancies that express CD 19 and CD22. The goal of developing this bispecific CAR is to reduce the risk of relapse through antigen loss. The investigators used the NCI CD19 construct FMC63 and the NCI CD22 construct m971 on a 4-1BB backbone to develop the bispecific CARs using the proprietary CliniMACS Prodigy platform.

Patients ages 3 through 30 with pretreated CD19/CD22-positive ALL or NHL were infused with the bispecific CAR T cells following lymphodepletion with chemotherapy; 13 out of the 15 patients enrolled were treated with one of the three doses tested.
The bispecific CAR T-cell therapy was well tolerated with reversible toxicity. Cytokine release syndrome (CRS) of grade 3 or higher occurred in about 15 percent of patients, Shalabi noted. One patient had reversible neurotoxicity.
Five of 12 evaluable patients had minimal residual disease (MRD)-negative complete responses. Notably, four of the five patients with complete response did not receive prior CAR T-cell therapy. CAR T-cell expansion was seen in all patients, and the cells persisted for a median of 45.6 days. Two of the five responding patients had disease relapse at 123- and 265-days post infusion, respectively, while the rest of them remained in remission at a median of seven months.
“Early experience with the bispecific CAR T cells demonstrates clinical activity with limited CRS and limited neurotoxicity,” Shalabi concluded. “We have seen discrepant results between marrow extramedullary disease, suggesting potentially limited CAR T-cell trafficking to sites of extramedullary disease.” This could potentially be overcome with higher doses of the treatment, she added.
Off-the-shelf, universal CAR T cells tested in a clinical trial
Xinxin Wang, from Gracell Biotechnologies Co., Ltd., in Shanghai, China, discussed the development and testing of TruUCAR GC027, a universal CAR T-cell therapy to treat patients with relapsed/refractory T-cell ALL (R/R T-ALL). This highly aggressive disease comprises about 20 to 25 percent of adult ALL and 12 to 15 percent of pediatric ALL, she said.

Wang highlighted the difficulties with developing treatments for T-ALL, as the disease shares common antigens with normal T cells. Further, potential contamination with lymphoblasts impedes the development of autologous CAR T-cell therapy. These challenges could be overcome by developing a universal CAR T, she said. GC027 was developed using the proprietary TruUCAR platform, and targets the protein CD7, present in more than 95 percent of T-ALL. Given that this is an allogenic product, potential graft versus host disease (GvHD) was avoided by editing out the expression of TCR through genomic disruption of the TRAC locus using CRISPR-Cas9 system. The CD7 locus was also edited out to avoid fratricide (self-killing) during CAR T-cell production.
Wang and colleagues enrolled patients ages 18 to 70 with R/R T-ALL that express CD7. All five patients treated with one of the three doses of GC027 had a complete response, and four of the responses were MRD-negative. All five patients had reversible and manageable CRS, Wang noted. No GvHD was observed. Grade 3 events included prolonged cytopenia, pulmonary infections, and febrile neutropenia. Flow cytometry and quantitative polymerase chain reaction studies revealed the expansion of GC027 in all patients.
“GC027 demonstrated very promising early response rate with a 100 percent complete response,” Wang said.
Combining CAR T-cell therapy and an immune checkpoint inhibitor to treat lymphoma
To test whether immune checkpoint blockade could augment the activity of CAR T cells, Caron A. Jacobson, MD, medical director of the Immune Effector Cell Therapy Program at Dana-Farber Cancer Institute, and colleagues conducted a phase I/II clinical trial, ZUMA-6, in which axicabtagene ciloleucel was combined with the anti-PD-L1 immune checkpoint inhibitor atezolizumab (Tecentriq) to treat patients with refractory diffuse large B-cell lymphoma. The primary endpoint of this study was dose-limiting toxicity and complete response rate.

The study showed that the combination had a manageable safety profile with no significant evidence of increased incidence of adverse events, and data were consistent with the observations from the clinical trial ZUMA-1, in which the CAR T-cell therapy was tested as monotherapy. In ZUMA-6, there were no deaths from CRS or neurologic events, and other adverse events were generally reversible. The persistence of CAR T cells beyond 28 days seemed greater in a small cohort of patients in the combination trial than that observed in ZUMA-1. The treatment responses with the combination were comparable to the responses in patients treated with axicabtagene ciloleucel alone; however, the study was not powered to test this outcome.
TIL therapy for non-small cell lung cancer
Ben Creelan, MD, a medical oncologist from H. Lee Moffitt Cancer Center, Tampa, Florida, described the development and testing of a TIL therapy for patients with immune checkpoint inhibitor-naïve non-small cell lung cancer in a phase I clinical trial.

Creelan and colleagues cultured T cells isolated from the lung tumors of 20 patients with cytokines, isolated the best cultures, mixed them with the original tumor cells and tested for tumor recognition, expanded the T cells, and infused them back into the patients. After TIL harvest, patients received four cycles of the anti-PD1 immune checkpoint inhibitor nivolumab (Opdivo).
Patients whose tumors responded to nivolumab continued the treatment, while those whose tumors progressed were switched to one infusion of TIL therapy, followed by IL-2 therapy, chemotherapy, and maintenance nivolumab for up to a year. Of the 12 evaluable patients who received TIL therapy, two patients had complete response, one had partial response, and three had unconfirmed partial response. One patient whose tumor showed pseudoprogression on radiological evaluation had a pathological response.
Notably, the traditional markers of benefit with immune checkpoint inhibitors, such as PD-L1 and tumor mutational burden did not seem to predict for benefit with TIL therapy. Whole-exome sequencing and RNA sequencing studies revealed that antigen-specific T cells comprised 21 percent of the infused TILs, but this needs further confirmation, Creelan noted.
“Lung cancer TIL therapy is feasible and expanded in 95 percent of patients,” concluded Creelan. “Clonotype and phenotype analysis suggested persistence of transferred TILs.”
Gene editing and bioinformatics platforms aid new avenues for adoptive cell transfer

Davide Bernareggi, PhD, a postdoctoral scholar from the University of California, San Diego, talked about using a CRISPR-Cas9 gene editing-based screening system to identify novel molecular targets that regulate natural killer (NK) cell-mediated killing of solid tumors. Bernareggi and colleagues identified genes that could modulate the sensitivity of glioblastoma stem cells and meningioma cells to NK-cell activity. They identified the protein CHMP2A as a new target to regulate NK-cell activity in glioblastoma stem cells and head and neck squamous cell carcinoma cells.

Edward Samuel, PhD, from University College London, discussed the development of a personalized autologous clonal neoantigen T-cell therapy for the treatment of solid cancers using the proprietary VELOS manufacturing platform. This process, which incorporates the proprietary PELEUS bioinformatics platform, was used to generate personalized T-cell products that could be directed at multiple cancer neoantigen targets. This could potentially be used to treat a variety of solid tumors, according to Samuel. Two clinical trials have been initiated to test this approach, for patients with non-small cell lung cancer and melanoma, in the United Kingdom.
Discussant of the two studies, Theodore Roth, MD/PhD student at the University of California, San Francisco, said, “Even though cellular therapies have been in development and in clinical practice for over 30 years, cumulatively, only [about] 1,000 patients have been treated. We have to ask ourselves, how is it that we are going to expand the accessibility of cellular and gene therapies to a much greater number of patients who may be able to benefit?”

Roth noted that this problem can potentially be solved by embracing the power of pooled genetic screens and selection, as demonstrated by the two presentations. He added that pooled genetic screening approaches can potentially accelerate the process of discovery and development of new cellular therapies, ultimately reducing the cost and increasing the efficacy of this type of cancer treatment.



