Editors’ Picks: August Highlights from the AACR Journals
This month, the editors of the AACR’s journals have decided to feature a clinical trial evaluating prolonged treatment with ibrutinib for patients with chronic lymphocytic leukemia/small lymphocytic lymphoma; a study investigating the impact of immune checkpoint inhibition on COVID-19 severity in patients with lung cancer; and a study examining the effects of long-term treatment with gemcitabine on the pancreatic cancer microenvironment, among other studies. As always, these articles are freely available online for a limited time.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Fecal immunochemical test (FIT) is one of several screening methods for colorectal cancer and is recommended to be completed annually for individuals over the age of 50 who are at average risk. Most studies examining adherence to FIT screenings have only measured the completion of one FIT and have not measured adherence to recommended follow-up procedures or to repeat FITs in subsequent years. In this study, the authors measured adherence to FIT in three different ways: (1) by completion of FIT within 9-15 months of a prior FIT, (2) by yes/no patterns of whether a FIT was completed in 9-15 month intervals, and (3) by the amount of time that subjects were up-to-date on their screening. The study followed 18,257 individuals over four rounds of screening. The authors found that adherence results varied by measure. When measured by method 1, they found that 15.8 percent of patients who had a normal FIT in round 1 completed a FIT in round 2. Method 2 showed that the most common yes/no pattern was yes-no-no-no, meaning that most patients completed a FIT only in the first year; 9.4 percent of patients completed a FIT in two consecutive years. In contrast, the adherence rate as measured by method 3 was 52.3 percent. The authors conclude that adherence rates vary widely based on how they are measured, suggesting the need for additional research to better approximate screening adherence. This article was highlighted in the August issue.
Journal: Cancer Immunology Research
Lymph nodes contain different subpopulations of stromal cells, which can contribute to tumor progression following metastasis of cancer cells into lymph nodes. The stromal cell subpopulations that respond to lymph node metastasis have not been defined. Here, the authors identified novel stromal cell subpopulations in melanoma-infiltrated lymph nodes. They found that melanoma-infiltrated lymph nodes contained fibroblastic reticular cells expressing T-cell modulating cytokines, chemokines, and adhesion molecules, as well as a previously unidentified population of CD34-positive stromal cells that produce the extracellular matrix. The transcription profiles of these subpopulations differed from each other. In addition, these populations were distinct from those found in normal lymph nodes. The authors also identified novel pericyte populations in the walls of high endothelial venules, small vessels, and large vessels in melanoma-infiltrated lymph nodes. The authors conclude that melanoma infiltration induces changes in lymph node stromal cell subpopulations. This article was featured on the cover of the August issue.
Journal: Clinical Cancer Research (August 1 issue)
While treatment with PARP inhibitors is standard of care for women with high-grade serous ovarian cancer (HGSOC), how to best treat those patients who develop resistance to PARP inhibition remains an area of active research. In this proof-of-concept clinical trial, researchers investigated the combination of the PARP inhibitor olaparib (Lynparza) with the VEGF inhibitor cediranib (Recentin) in patients with HGSOC whose cancer progressed following previous treatment with a PARP inhibitor. The 34 women enrolled in the trial were categorized into three cohorts: those who were sensitive to platinum treatment following PARP inhibition; those who were resistant to platinum treatment following PARP inhibition; and those in the exploratory cohort, whose cancer progressed following PARP inhibition and progressed again following standard chemotherapy, regardless of platinum sensitivity. Objective responses were observed in 0 percent, 20 percent, and 8 percent of patients in the platinum-sensitive, platinum-resistant, and exploratory cohorts, respectively; 16-week progression-free survival rates were 55 percent, 50 percent, and 39 percent, respectively. When the researchers evaluated paired biopsy samples taken before and after initial treatment with a PARP inhibitor, they found that patients with reversion mutations in homologous recombination genes and/or upregulation of the ABCB1 gene had poor outcomes following the olaparib-cediranib combination. These results suggest that the mechanism of PARP inhibitor resistance can affect sensitivity to olaparib-cediranib treatment in patients with HGSOC whose cancer has previously progressed following treatment with a PARP inhibitor. This article was highlighted in the August 1 issue.
Journal: Clinical Cancer Research (August 15 issue)
Bruton’s tyrosine kinase (BTK) is required for B-cell signaling, is overexpressed in many B-cell malignancies, and has therefore been exploited as a therapeutic target. In this study, the longest follow-up for the selective BTK inhibitor ibrutinib (Imbruvica) as a single agent is described. The researchers evaluated data from patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) enrolled in the phase Ib/II PCYC-1102 trial and the extension study PCYC-1103 who received daily ibrutinib in the first-line or relapsed/refractory setting. The overall response rate was 89 percent, with a complete response rate of 35 percent and 10 percent in the first-line setting and relapsed/refractory setting, respectively. Estimated seven-year progression-free survival rate and overall survival rate in the first-line setting was 83 percent and 84 percent, respectively. In the relapsed/refractory setting, the estimated seven-year progression-free survival rate and overall survival rate was 34 percent and 55 percent, respectively. Adverse events (AEs) of grade 3 or higher that occurred in more than 15 percent of patients were hypertension, pneumonia, and neutropenia; these grade 3 AEs generally declined over time, with the exception of hypertension. These results suggest that prolonged single-agent ibrutinib treatment is safe and efficacious for patients with CLL/SLL. This article was highlighted in the August 15 issue.
Journal: Cancer Discovery
Impact of PD-1 Blockade on Severity of COVID-19 in Patients with Lung Cancers
associations between PD-1 blockade
and COVID-19 severity in patients
with lung cancer. The study was featured
on the issue’s cover, shown here.
The effect of immune checkpoint inhibition on coronavirus disease 2019 (COVID-19) remains unclear. Although the amplification of the immune response after immune checkpoint inhibition might help suppress viral infection, there is concern that the increased immune activity might also enhance the severity of COVID-19. In this study, the authors examined how PD-1 immune checkpoint inhibition affected the severity of COVID-19 in patients with lung cancer. The study included 69 patients with lung cancer who had confirmed COVID-19; 41 of these patients had previously received PD-1 blockade. The authors compared the rates of hospitalization, intensive care unit utilization, intubation, transition to a do-not-intubate status, and death between patients who had previously received PD-1 blockade and those who had not. They found no significant differences in COVID-19 severity, even after adjusting for smoking history and gender. The authors conclude that PD-1 immune checkpoint inhibition does not affect the severity of COVID-19. This article was highlighted and featured on the cover of the August issue.
Journal: Cancer Research (August 1 issue)
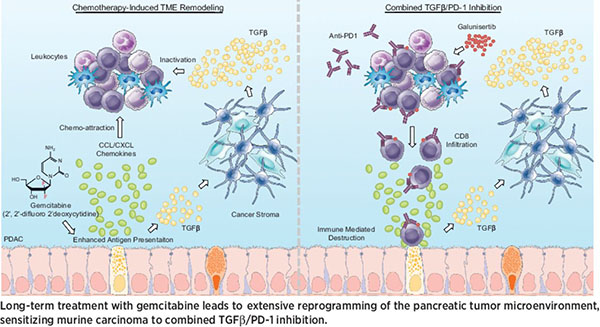
The median survival time for patients diagnosed with pancreatic cancer is six to 12 months, and there are no approved treatments for patients who progress on chemotherapy. In this study, the authors examined the impacts of long-term gemcitabine treatment in order to identify potential therapeutic strategies for pancreatic cancers that do not respond to chemotherapy. Using a combination of mouse models, patient-derived xenografts, and established cell lines, the authors found that gemcitabine increased antigen presentation, immune checkpoint expression, CCL/CXCL chemokine synthesis, and TGFβ-associated signals. Secretion of CCL/CXCL chemokines and TGFβ altered the tumor stroma and led to gemcitabine resistance and additional TGFβ synthesis. Combination treatment with gemcitabine and anti-PD-1 therapy did not affect outcomes in mice unless TGFβ signaling was inhibited by galunisertib. In the context of TGFβ inhibition, gemcitabine in combination with anti-PD-1 therapy led to a strong T-cell response, a decrease in tumor burden, and improved survival in mice. The authors propose that multi-pronged treatment consisting of TGFβ inhibition, gemcitabine, and immune checkpoint inhibition may be a therapeutic option for chemorefractory pancreatic cancers. This article was featured on the cover of the August 1 issue, and a related commentary can be found here.
Journal: Cancer Research (August 15 issue)
Senescent cells, which inherently prevent neoplastic events, paradoxically develop an inflammatory secretome, termed the senescence-associated secretory phenotype (SASP), which is implicated in cancer. In this study, the authors demonstrate that stromal senescent cells secrete small extracellular vesicles (sEV), which enhanced the aggressiveness of prostate cancer cells and altered their expression profile, notably through increased expression of ABCB4, a gene involved in drug efflux. The researchers also found that loss of the enzyme SIRT1 resulted in increased sEV production in senescent stromal cells, and that targeting SIRT1 with an agonist restrained sEV secretion in senescent stromal cells and diminished the chemotherapeutic resistance of co-cultured prostate cancer cells. Further, analysis of tissue samples taken from patients with prostate cancer treated with chemotherapy revealed a significant association between stromal SIRT1 expression and patient survival. These results identify a mechanism that supports the pathological activities of senescent cells and provides a potential avenue for therapeutic intervention. This article was highlighted and was featured on the cover of the August 15 issue. A related commentary can be found here.
Journal: Molecular Cancer Research
Targeting RET Kinase in Neuroendocrine Prostate Cancer
The use of second-generation antiandrogen therapies has proven efficacious for patients with recurrent or metastatic castration-resistant prostate cancer; however, this approach correlated with an increase in the incidence of aggressive variant prostate cancer (AVPC) that has lost dependence on androgen receptor (AR) signaling. A subset of AVPC tumors, classified as neuroendocrine prostate cancer (NEPC), are highly aggressive and have no curative treatment options. Recent evidence suggests that kinase signaling may be an important driver of NEPC, yet specific signaling pathways have not been identified or exploited as a therapeutic target. To identify altered kinase signaling pathways that are unique to AR-independent prostate cancers, this study evaluated the phosphoproteome of several AR-dependent and AR-independent prostate cancer cell lines. The researchers found that the receptor tyrosine kinase RET and several of its downstream effectors were enriched and activated in the AR-independent cell lines. Further, the researchers found that expression of RET was upregulated in patients with NEPC in multiple clinical datasets. Finally, the genetic knockdown or pharmacological inhibition of RET reduced tumor growth in NEPC models. The authors conclude that targeting RET may be a viable approach for patients with NEPC with high RET expression. This article was highlighted in the August issue.
Journal: Molecular Cancer Therapeutics
The Indenoisoquinoline LMP517: A Novel Antitumor Agent Targeting Both TOP1 and TOP2
Topoisomerase I inhibitors derived from camptothecin, such as irinotecan (Camptosar) and topotecan (Hycamtin), are approved for the treatment of several cancer types but are limited by their short plasma half-life, drug efflux, and, in the case of irinotecan, severe toxicity. To overcome these limitations, alternative topoisomerase I inhibitors, derived from indenoisoquinoline, were developed, and three such drugs are currently being tested in clinical trials. Second-generation indenoisoquinolines, termed fluoroindenoisoquinolines, have recently been described, and the fluoroindenoisoquinoline LMP517 is the focus of the current study. Compared with its parent compound LMP744, LMP517 showed enhanced antitumor activity in a small cell lung cancer xenograft model. Further, the researchers found that LMP517 inhibited both toposimerase I and topoisomerase II in both biochemical assays and in cell lines. In addition, LMP517 was found to target cells independently of their position in the cell cycle, unlike camptothecin-derived topoisomerase I inhibitors. The authors conclude that LMP517 is a dual topoisomerase inhibitor with therapeutic potential. This article was highlighted in the August issue.
Journal: Cancer Prevention Research
Sulforaphane is a byproduct found in broccoli sprouts that has shown chemopreventive activity against prostate cancer in several preclinical models. Sulforaphane can induce autophagy or apoptosis, but the induction of autophagy inhibits apoptosis. The mechanism underlying sulforaphane-induced autophagy and its inhibition of apoptosis remains unclear. In this study, the authors examined the role of lysosome-associated membrane protein 2 (LAMP2), which has been previously implicated in the regulation of autophagy in many cancers and is a known prognostic factor for prostate cancer. They found that sulforaphane treatment led to a dose-dependent increase in LAMP2 protein and mRNA levels in prostate cancer cell lines, as well as the induction of several autophagy-related genes. Depletion of LAMP2 led to the activation of proapoptotic proteins and increased apoptosis in cells treated with sulforaphane. Together, the results suggest that sulforaphane treatment promotes autophagy and inhibits apoptosis through induction of LAMP2 in prostate cancer cells.



