Examining Combination Therapies for Pancreatic Ductal Adenocarcinoma
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive disease with a grim prognosis. While early-stage PDAC may be removed by surgery, PDACs that have spread beyond the pancreas are generally deemed inoperable. Unfortunately, 80 to 90 percent of patients are diagnosed only after the cancer has spread, which limits the treatment options available to these patients. In addition, PDAC is resistant to many existing cancer therapies, due in part to an immunosuppressive tumor microenvironment.
Some patients with advanced PDAC may be treated with radiation therapy, either alone or in combination with other therapies. Conventional radiation therapy delivers frequent small doses of radiation over five to six weeks. In contrast, an emerging strategy known as stereotactic body radiation therapy (SBRT) delivers higher doses of radiation in fewer fractions and can target the tumor more precisely to decrease the damage to surrounding normal tissue. Radiation-induced cell death can expose tumor antigens to activate an antitumor immune response.

While SBRT has shown promise for the treatment of PDAC, additional work is needed to optimize the therapeutic efficacy, according to Scott Gerber, PhD, associate professor at the University of Rochester Medical Center. “Our previous work demonstrated that while SBRT monotherapy can initiate an antitumor immune response, we need other therapies to magnify and sustain the response,” Gerber explained. “As a result, there is a growing thought that combination therapy will be more efficacious than monotherapy to treat cancer.”
In two preclinical studies, Gerber and colleagues have explored the efficacy of combining SBRT with chemotherapy or immunotherapy for the treatment of pancreatic tumors.
SBRT and FOLFIRINOX
In a study published earlier this year in Cancer Immunology Research, a journal of the American Association for Cancer Research, Gerber and colleagues examined the efficacy of combining SBRT with FOLFIRINOX (FX), a chemotherapy regimen that is often used to treat patients with PDAC.
“We already knew that the magnitude of the antitumor response is related to the magnitude of the initial immune response,” said Gerber. “We hypothesized that combining SBRT with chemotherapy would lead to a greater immune initiation, and therefore, a greater antitumor response.” The combination of radiation and chemotherapy was previously shown to induce immunogenic cell death, which results in activation of the immune response.
“In this study, we used a modified FX dose (mFX), which is essentially less chemotherapy,” added Gerber. “This means less toxicity, and since chemotherapy can adversely affect the immune system, using a lower dose of FX could bolster the antitumor immune response.”
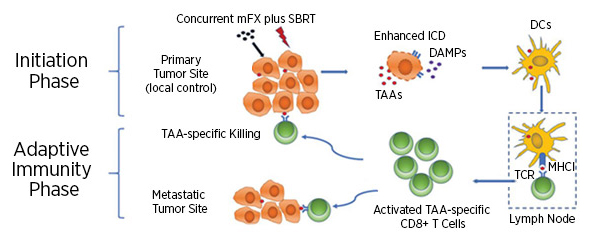
Using preclinical models of PDAC, Gerber and colleagues demonstrated that mice treated with concurrent SBRT and mFX had significantly greater survival compared to mice treated with either therapy alone. Examination of tissue from the treated mice demonstrated that the combination of SBRT and mFX led to greater tumor cell death, higher expression of immunogenic cell-surface markers, and increased secretion of the damage-associated molecular pattern (DAMP) HMGB1, indicating increased immunogenic cell death. Furthermore, Gerber and colleagues observed increased recruitment of dendritic cells, which can activate CD8+ T cells and stimulate adaptive antitumor immunity. Depletion of dendritic cells or CD8+ T cells abrogated the therapeutic efficacy of the SBRT and mFX combination, suggesting that these cell types are necessary for the greater antitumor effect observed with the combination. Using a hemisplenic mouse model of metastasis, Gerber and colleagues demonstrated that mice treated with the combination therapy had fewer metastases to the liver, which is the most common site of metastasis for PDAC. The authors propose that the SBRT and mFX combination therapy activates local and systemic antitumor responses that target the primary tumor and any developing metastases, respectively.
“Previous studies have demonstrated therapeutic benefit to combining SBRT with chemotherapy, and we have now shown that the modified FX dose, which has less toxicity, also enhances the therapeutic effect when combined with SBRT,” said Gerber. “Additionally, we showed that the magnitude of the antitumor response is correlated with the release of immunogenic factors, such as HMGB1. We therefore expect that the combination of therapies that stimulate the greatest release of these factors are likely to result in better antitumor responses.”
SBRT and IL-12 microsphere immunotherapy
In another study, which was published in Cell Reports in October 2019, Gerber and colleagues combined SBRT with interleukin-12 (IL-12) microsphere immunotherapy in preclinical mouse models of PDAC. While radiation-induced cell death can expose tumor antigens that help activate the immune response, it can also attract immunosuppressive cell types. Gerber and colleagues hypothesized that adding IL-12 microsphere immunotherapy would help reverse the immunosuppressive effects of SBRT and enhance the therapeutic efficacy.
Consistent with their hypothesis, they found that the combination therapy led to significant reductions in tumor burden and significant increases in survival when compared to each therapy alone. The combination of SBRT and IL-12 led to significantly higher levels of intratumoral IL-12, interferon-gamma (IFNy), and CXCL10 and significantly higher levels of IFNγ-secreting immune cells. Furthermore, the combination therapy prevented the recruitment of certain immunosuppressive cell types that occurs with SBRT alone. The therapeutic benefits of the combination therapy were found to be dependent on IFNγ. In addition, Gerber and colleagues observed that the combination of SBRT and IL-12 microsphere immunotherapy led to a systemic antitumor effect that resulted in long-term survival of treated mice. Similar to the Clinical Immunology Research paper, the authors used a hemisplenic mouse model to demonstrate that the combination therapy led to a significant decrease in liver metastases.
Together, results from both studies indicate that antitumor efficacy of SBRT can be enhanced by adding concurrent modified FX therapy or IL-12 microsphere immunotherapy. “We demonstrated that these combination therapies reduced tumor burden and stimulated a greater systemic immune response that was capable of controlling and even eliminating distal disease (metastases),” said Gerber. “Since many cancer patients ultimately succumb to metastatic disease, therapies that are effective against both the primary and distal tumors would be greatly advantageous.”
Gerber and colleagues are also exploring combining SBRT with other therapies, such as checkpoint inhibitors and therapies that target immunosuppressive myeloid cells or intratumoral nerves, which they have shown can be immunosuppressive. They are also examining the efficacy of SBRT for other cancers using preclinical models.
“Our goal for the immediate future is translating our work into the clinic,” said Gerber. “We are working with the FDA to move many of these promising therapies into clinical trials and are also working diligently to optimize our therapies to combat metastatic disease.”