AACR Annual Meeting 2021: How Do Diet and Ancestry Affect Cancer Development?
Many patients facing a cancer diagnosis ask their doctors a simple question: “What should I eat?”
That elemental question has perplexed scientists for centuries. In a stirring discussion during the Opening Plenary session of the virtual AACR Annual Meeting 2021, Matthew Vander Heiden, MD, PhD, director of the Koch Institute for Integrative Cancer Research at MIT, and Karen Vousden, PhD, chief scientist of Cancer Research UK, reviewed some of the mechanisms behind diet’s role in cancer.
Questions about the connection between diet and disease date as far back as the 4th century B.C., when Hippocrates reportedly advised, “Let food be thy medicine.” In the mid-1400s, Bartolomeo Platina published a text that is generally translated as “On Honorable Pleasure and Good Health.” The text is considered the first cookbook (recipes include eel pie with rosewater and raisins!), but it also discussed Hippocratic theories that various foods affected the “humours” of the body (for example, mushrooms would make one melancholic).
In the ensuing centuries, dietary recommendations have been subject to trends, shifting rapidly and producing contradictory information, Vander Heiden and Vousden said. The general public often expresses confusion over what to eat, and those with a cancer diagnosis may feel extra urgency to tailor their diet to fight against their cancer.
There are, indeed, relationships between food and cancer. But they are rarely simple, nor are they the same for all patients or all types of cancer. In their plenary talk, Vander Heiden and Vousden reviewed several examples of how food or compounds within certain foods affect cancer. For example, Vousden discussed research that showed that mice that were fed diets low in the amino acids serine and glycine experienced slower tumor development and longer survival than mice fed an ordinary diet.
Meanwhile, Vander Heiden talked about research conducted by a postdoctoral student in his lab, in which a low-calorie diet was compared with the trendy ketogenic diet. While only the low-calorie diet slowed tumor growth in mice, the ketogenic diet altered lipids, leading to changes in tumor metabolism. The effects differed between palm oil and soybean oil, underscoring for Vander Heiden the difficulty of making generalized statements about diet and cancer.
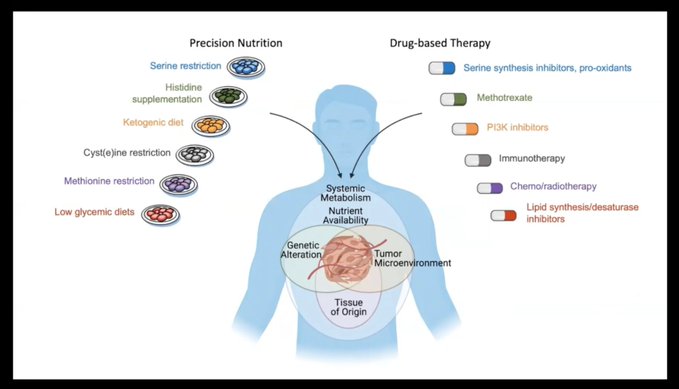
“It’s not that one source is always good or always bad,” he said. “We have to consider the full context of any dietary recommendation.”
Vousden agreed. While future research on diet and cancer may ultimately lead to “precision nutrition, only by fully understanding all the factors will we be able to determine what combination of dietary recommendations might be most helpful,” she said.
Also during the Annual Meeting, a Major Symposium titled “Diet, Clock, and Cancer” featured preclinical studies on the effects of restriction of specific nutrients and time-restricted eating on tumor growth. This session is available on demand for Annual Meeting registrants, and also appeared in AACR News, the official digital news source of the AACR Annual Meeting.
HOW ANCESTRY AFFECTS CANCER
In another presentation in the Opening Plenary, Melissa B. Davis, PhD, of Weill Cornell Medical College discussed “Ancestry and cancer disparities: Genomic sequencing in diverse populations.” Davis noted that while socioeconomic factors account for some of the disparity in outcomes between Black Americans and white Americans with cancer, tumor characteristics account for about 24 percent of the gap.
In order to reduce disparities, researchers need more information about the genetic differences between tumors in various racial and ethnic minority groups. But even the process of gathering information through genomic sequencing is markedly different for Blacks than whites, she said. Most large genomic studies have predominantly enrolled white patients. Because this leads to paucity of data on the mutations encountered in the tumors of Blacks and other racial minority groups, they are often classified as “variants of unknown significance.” This can disqualify a patient from participating in clinical trials, as their tumor mutations are thought to be “unactionable,” she said.
In an effort to gain more information on ancestry-based disparities, the New York Genome Center launched the Polyethnic-1000 initiative. Using data from the International Center for the Study of Breast Cancer Subtypes, where Davis is the scientific director, researchers have been able to expand their understanding of certain genetic components of triple-negative breast cancer, a deadly subtype that is more prevalent in African American women than in white women. Among these genetic variants is the Duffy-Null allele, a common factor in triple-negative breast cancers diagnosed in women with west African ancestry. Davis and colleagues have been able to evaluate links between the genetic factors that originated in west Africa and African American women’s poorer outcomes from triple-negative breast cancer. This allele is one piece of evidence that common ancestry affects outcomes from this deadly cancer type, Davis said.
“For triple-negative breast cancer, genetic ancestry may be associated with a novel tumor landscape. This is one of the stories that’s starting to emerge: that there’s a pipeline of shared genetic characteristics,” she said.
Davis added that current Polyethnic-1000 projects, focusing on prostate, bladder, lung, and endometrial cancers, will also drill deeply into ethnic differences and immigration patterns to further elucidate the role of ancestry in cancer disparities.
OTHER HIGHLIGHTS FROM THE OPENING PLENARY
Jonathan S. Weissman, PhD, of the Whitehead Institute in Cambridge, Massachusetts, discussed a new strategy for following the life cycle of a tumor, from its earliest initiation through metastasis. Using the genome-editing tool CRISPR-Cas9, he and colleagues have developed a “molecular flight recorder” technology that allows researchers to record the history of a tumor in its DNA, reconstruct its phylogenetic tree, and follow its evolution and metastasis.
Weissman shared several examples of how he and colleagues have engineered cancer cells in mice to include a neutral part of DNA, or “scratchpad,” which can be targeted by CRISPR-Cas9 over time during the evolution of the tumor. The scratchpad has allowed them to trace tumors at their earliest stage, and to watch as some become aggressive. So far, this technology has allowed the researchers to observe a high degree of heterogeneity in metastatic states. Going forward, Weissman expects the scratchpad to allow researchers to detect the earliest stages of therapeutic resistance and to observe how tumor cells interact with various molecular events. The technology “reveals a hidden dimension in tumor biology,” Weissman said.
Mark A. Dawson, MD, PhD, of the Peter MacCallum Cancer Center, discussed “Epigenetic mechanisms of malignant clonal dominance and immune evasion.” Dawson shared evidence that not all cancer behavior can be explained by genetic factors. In fact, cancer cell clones with the exact same mutation may behave differently within the same cancer patient, he said.
“Cell behavior cannot simply be explained by the cancer genome, and really for us to achieve our ambition of precision medicine, we need to study and further develop approaches to tackle non-genetic mechanisms of clonal dominance,” he said.
In a presentation titled “Igniting an immune response with cGAS,” Zhijan James Chen, PhD, of UT Southwestern Medical School, addressed the fact that immunotherapy, while highly successful in some patients, does not work for all patients. He discussed his research that led to the discovery of cGAS, a DNA-sensing enzyme that plays a role in immunotherapy response.
Chen explained that when cGAS senses DNA, it produces a small molecule called cyclic GMP-AMP (cGAMP), which then activates the STING protein, leading to induction of type-I interferons and other cytokines. Chen said this pathway is important for innate antitumor immune response.
“By better understanding this pathway and by harnessing the power of the cGAS-STING pathway … new therapies can be developed to benefit patients who currently do not have good options,” he said. Cancer Research Catalyst will take a closer look at cGAS in a future post.