Editors’ Picks for July
As we welcome in August, it’s time to look back at some of the top articles published in the nine AACR journals during the month of July. These 11 “must-read” articles, selected by the editors, include three clinical trials, an analysis of the effects of exercise on antitumor immune responses, and an investigation into the use of hypothermia to treat p53-mutant tumors, among others. As always, all of the highlighted articles are free to read for a limited time.
Journal: Cancer Discovery
Insertion mutations in exon 20 of the epidermal growth factor receptor (EGFR) gene account for 4-12 percent of EGFR mutations in non-small cell lung cancers (NSCLC). Existing EGFR inhibitors target more common EGFR mutations and are less effective against NSCLCs harboring EGFR exon 20 insertion mutations. A preclinical study also published in this issue of Cancer Discovery found that mobocertinib, an inhibitor that targets EGFR exon 20 insertion mutations, suppressed cancer growth in NSCLC models. Here, the authors conducted a phase I/II clinical trial to evaluate the safety and efficacy of mobocertinib in patients with previously treated NSCLC. The recommended phase II dose and maximum tolerated dose were determined to be 160 mg daily. Among the 136 patients treated at this dose, the most common treatment-related adverse events were diarrhea, nausea, rash, and vomiting. Of the 28 patients with NSCLC harboring EGFR exon 20 insertion mutations who were treated at the recommended phase II dose, 12 patients (43 percent) had an objective response. The median duration of response was 14 months, and the median progression-free survival was 7.3 months. The authors conclude that mobocertinib showed clinical efficacy in patients with EGFR exon 20 insertion mutations and had a safety profile consistent with other EGFR inhibitors. This article was highlighted in the July issue, and a related commentary can be found here.
Journal: Clinical Cancer Research (July 1 issue)
Gene duplications or activating mutations in anaplastic lymphoma kinase (ALK) drive a variety of cancers, including a subset of neuroblastomas. Although the ALK inhibitor crizotinib has been approved for treatment of specific types of lung cancer and lymphoma, its efficacy in neuroblastoma has yet to be determined. In this study, researchers treated 20 pediatric neuroblastoma patients, whose tumors relapsed on other therapies and had a mutated and/or amplified ALK gene, with crizotinib. Fifteen percent of patients’ tumors had a partial or complete response, and all responsive tumors harbored the same ALK mutation (Arg1275Gln). This suggested to the authors that other common ALK mutations (such as Phe1174Leu and Phe1174Val, also evaluated in this study) may exhibit innate resistance to crizotinib. This finding supported data from preclinical studies showing that these mutations have a high affinity for ATP, limiting the extent to which they interact with crizotinib. This study was highlighted in the July 1 issue, and a related commentary can be found here.
Journal: Cancer Prevention Research
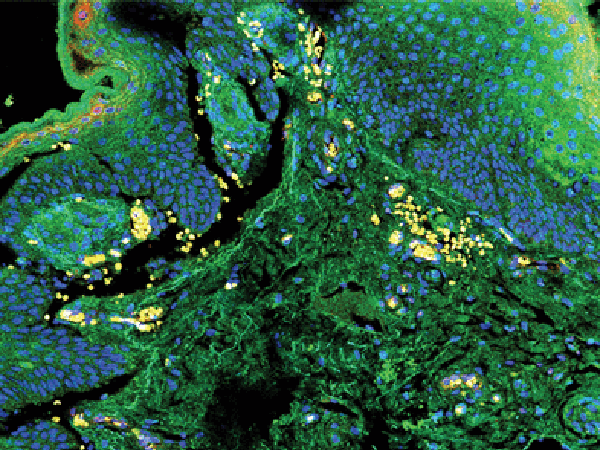
Dopamine regulates several physiologic processes, including suppression of VEGF-mediated angiogenesis in malignant tumors; however, its role in premalignant lesions remains unclear. In this study, the authors examined the role of dopamine in the development and progression of ultraviolet B (UVB)-induced premalignant lesions. They found that the stimulation of dopamine D2 receptors in UVB-induced skin lesions significantly reduced lesion number, lesion burden, and progression to malignant squamous cell carcinoma in mice. An agonist of the D2 receptor inhibited the expression of VEGF-dependent proangiogenic genes in vitro and in vivo, suggesting that dopamine’s impact on angiogenesis is dependent on D2 receptors. The authors conclude that dopamine may inhibit the progression of premalignant UVB-induced skin lesions to squamous cell carcinoma. This article is featured on the cover of the July issue.
Journal: Molecular Cancer Research
EWS-FLI1 and Menin Converge to Regulate ATF4 Activity in Ewing Sarcoma
Most Ewing sarcomas—cancers of the bone or soft tissue commonly diagnosed in pediatric patients—are driven by EWS-FLI1 gene fusions, which can facilitate widespread metabolic reprogramming that feeds tumor cells. Increased serine synthesis represents one such adaptation, but the mechanism is currently unknown. In this study, researchers identified the stress response gene ATF4 as a crucial mediator of this process. They showed, in cell models of Ewing sarcoma, that EWS-FLI1 directly stimulates expression of ATF4, and that knockdown of ATF4 decreases cell proliferation and expression of serine biosynthesis genes. In other tumor types, serine biosynthesis is driven by the scaffolding protein menin, but whether or not menin activity is dependent upon ATF4 is currently unknown. Here, researchers showed that knockdown of menin completely blocks ATF4 expression and decreases serine biosynthesis in cell line models of both Ewing sarcoma and mixed-lineage leukemia, accompanied by a modest decrease in cell proliferation. The authors speculate that menin may be a promising therapeutic target for diseases driven by serine biosynthesis, including EWS-FLI1-mutated Ewing sarcomas. This study was highlighted in the July issue, during Sarcoma Awareness Month.
Journal: Cancer Immunology Research
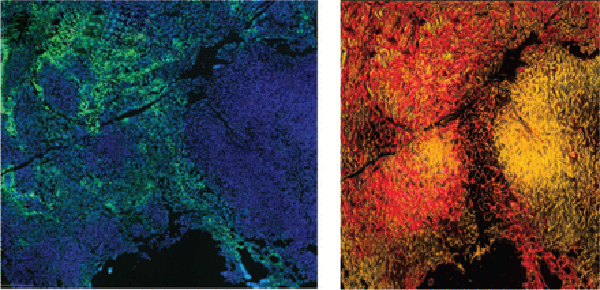
Exercise is associated with several health benefits, including reduced morbidity and mortality in patients with some types of cancer. The impact of exercise on antitumor immune responses remains unclear. Here, the authors utilized mouse models of breast cancer to explore the effect of exercise on tumor growth, the tumor microenvironment, and the response to immune checkpoint blockade (ICB). They found that mice that underwent exercise training had delayed tumor growth, normalized blood vasculature, and greater infiltration of CD8+ T cells with enhanced effector function in the tumor microenvironment. Furthermore, exercise training sensitized ICB-refractory breast cancers to ICB. T-cell recruitment and antitumor effects were abrogated in mice lacking the Cxcr3 gene, suggesting that the CXCL9/CXCL11-CXCR3 pathway may help promote antitumor responses upon exercise. Together, the results indicate that exercise can normalize tumor vasculature, influence the tumor microenvironment, enhance antitumor immune responses through CD8+ T cells and CXCR3, and sensitize cells to ICB. This article was featured on the cover of the July issue. This study was presented as a poster at the AACR Annual Meeting 2021.
Journal: Cancer Research (July 15 issue)
Hypothermia Effectively Treats Tumors with Temperature-Sensitive p53 Mutations
Approximately half of all tumors have an inactivating mutation in the tumor suppressor p53, most often in the DNA binding domain. Although efforts to correct mutant p53 structure or to express wild type p53 have faced many challenges, researchers have shown that 10 to 15 percent of p53 mutations are temperature sensitive, meaning the mutant protein can bind DNA at 32 degrees Celsius, but not at the normal body temperature of 37 degrees Celsius. In this study, researchers showed that tumor cells with temperature-sensitive p53 mutations proliferated more slowly at 32 degrees Celsius, but that this was not true of cells with non-temperature-sensitive mutations. Researchers also investigated a way to pharmaceutically induce hypothermia in mice using a drug that prevents the brain from regulating body temperature. They injected mice with temperature-sensitive mutant lymphoma or lung cancer cells and showed that tumor growth was inhibited during drug-induced hypothermia, performed six times for 24 hours each or five times for 32 hours each. When combined with DNA damaging chemotherapy, tumor size regressed, which continued in some cases after hypothermia treatment had ceased. This suggests hypothermia as a potential therapeutic option for patients whose tumors harbor temperature-sensitive p53 mutations. A related commentary can be found here.
Journal: Blood Cancer Discovery
Lysine Demethylase 5A Is Required for MYC-Driven Transcription in Multiple Myeloma
MYC is required for the growth of multiple myeloma cells; however, therapeutic targeting of MYC has thus far been challenging. The binding of MYC to transcriptional promoters is dependent on histone H3 trimethylation (H3K4me3), a histone mark associated with gene transcription. Because the enzyme lysine demethylase 5A (KDM5A) regulates H3K4me2 and H3K4me3, the authors of this study hypothesized that KDM5A may impact MYC-driven transcription in multiple myeloma. They found that KDM5 proteins, including KDM5A, were highly expressed in multiple myeloma cells, and that KDM5A depletion led to reduced growth of multiple myeloma cell lines. They also determined that KDM5A binds MYC at target genes and activates transcription by interacting with the positive transcription elongation factor b (PTEFb) complex. The authors found that KDM5 inhibition or KDM5A depletion increased H3K4me3 and decreased transcription of MYC target genes, suggesting that KDM5A promotes the activation of MYC target genes in multiple myeloma. Furthermore, they determined that KDM5A promoted transcription of MYC target genes through TFIIH- and PTEFb-dependent phosphorylation of RNA polymerase II. The authors propose that targeting KDM5A to reduce transcription of MYC target genes may be an effective therapeutic strategy for multiple myeloma. This study was also presented at the AACR Annual Meeting 2021; watch the presentation here.
Journal: Molecular Cancer Therapeutics
Gastrointestinal stromal tumors (GIST) are most commonly driven by activating mutations in the protein kinase KIT, the activity of which can be blocked by tyrosine kinase inhibitors such as imatinib. However, imatinib rarely results in tumor regression, and many tumors develop imatinib resistance by accumulating additional KIT mutations or by upregulating downstream elements of the MAPK pathway. Ripretinib, a newer KIT inhibitor, was designed to block activity of mutated KIT, and it evoked promising responses in the INVICTUS phase III clinical trial. The authors of this study investigated whether combining ripretinib with the MEK inhibitor trametinib could boost its efficacy and promote tumor regression. They showed that the combination could induce death of GIST cells, as well as systemic mastocytosis cells, and that it could block colony formation of both cell types. Further, when these cells were injected into mice, a high-dose combination of ripretinib and trametinib resulted in complete tumor regression in all 10 mice, compared with six of 10 mice treated with ripretinib alone. Tumors treated with the combination also did not relapse as quickly as ripretinib-treated tumors following treatment cessation. These results suggest that combining ripretinib with an inhibitor of the MAPK pathway may provide a new therapeutic option for patients with KIT-mutated tumors. This article was highlighted in the July issue.
Journal: Clinical Cancer Research (July 15 issue)
Some defects that help cancer cells grow and adapt result in the accumulation of DNA damage that must be kept in check by DNA repair pathways. The cell cycle regulator Wee1 provides the cell time to repair damage by inhibiting Cdk1 and Cdk2, crucial cell cycle drivers. In a previous phase I clinical trial, researchers found that some tumors of various types responded well to twice-daily adavosertib, a Wee1 inhibitor. Here, researchers aimed to determine whether a once-daily dose would suffice. Researchers treated 42 patients with 225, 250, 300, or 400 mg of adavosertib, once per day on days one through five and eight through 12 of a 21-day treatment cycle. The 300 mg per day dose was well-tolerated and selected as the recommended phase II dose. Six patients’ tumors—of ovarian or endometrial origin—had a partial response, which is similar to the response rate of twice-daily adavosertib. Patients whose tumors responded had increased expression of Cyclin E1, a gene that promotes cell cycle progression. The authors suggest that once-daily adavosertib may be sufficient for treatment, and that cyclin E1 may be a potential biomarker for adavosertib efficacy. This study was highlighted in the July 15 issue.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Solid Organ Transplantation and Survival among Individuals with a History of Cancer
Immune surveillance may help control cancer growth in patients with a prior history of cancer. Therefore, the authors of this study hypothesized that immune-suppressing medications administered to patients undergoing solid organ transplantation may increase the likelihood of cancer recurrence, and subsequent death, in individuals with a history of cancer. The authors examined data from the United States solid organ transplant registry and 13 cancer registries to compare survival between cancer survivors who did and did not undergo organ transplantation. The study included over 10 million patients with cancer; 0.05 percent of these patients underwent subsequent organ transplantation after a median of 5.7 years from cancer diagnosis. After adjusting for demographics, cancer stage, and time since cancer diagnosis, the authors found that organ transplantation was associated with reduced overall survival for most cancers, but not with cancer-specific survival for any cancer type. Furthermore, organ transplantation was associated with increased cancer-specific survival for patients with breast cancer, non-Hodgkin lymphoma, and multiple myeloma. The authors conclude that immunosuppression in organ transplant patients with a history of cancer is unlikely to negatively impact cancer-specific survival. This article was highlighted in the July issue.
Journal: Cancer Research (July 1 issue)
TIMP1 Triggers Neutrophil Extracellular Trap Formation in Pancreatic Cancer
Neutrophil extracellular trap (NET) formation has been shown to promote the progression of pancreatic ductal adenocarcinoma (PDAC), but the cellular mechanisms that promote NET formation are not well understood. By analyzing a dataset of human PDAC proteomes, the authors of this study observed that neutrophil activation correlated with expression of the TIMP1 protein, which is associated with poor prognosis in patients with pancreatic cancer. In cell culture experiments, TIMP1 expression led to NET formation in primary human neutrophils, and NET formation was dependent on the interaction of TIMP1 with the CD63 receptor and subsequent ERK signaling. In a mouse model of PDAC, TIMP1 contributed to NET formation, and depletion of TIMP1 or abrogation of NET formation prolonged mouse survival. Areas of high TIMP1 expression were found to colocalize with NETs in patient-derived PDAC tumors, and plasma levels of TIMP1 correlated with levels of NET markers in patients with PDAC. The authors utilized a combination of TIMP1, NETs, and CA19-9 levels to classify patients into prognostic subgroups. The authors conclude that TIMP1 may promote NET formation, and that TIMP1 expression may have potential as a prognostic biomarker in PDAC.



