Editors’ Picks: August Highlights from AACR Journals
For the month of August, the editors of the nine AACR journals have chosen to highlight results from two clinical trials, a study examining mutations that mediate acquired resistance to KRAS inhibition, and the development of a new method to distinguish acute myeloid leukemia from clonal hematopoiesis, among others. As always, the highlighted articles are freely available online for a limited time.
Journal: Cancer Discovery
Inhibitors targeting the oncogenic KRASG12C mutation, including sotorasib (Lumakras) and adagrasib, have undergone rapid clinical development in recent years. However, many patients acquire resistance to these inhibitors, a process that has been studied in cell and animal models but has yet to be comprehensively characterized in humans. The authors of this paper evaluated resistance mechanisms in a patient’s non-small cell lung cancer tumor that initially responded to adagrasib, then developed resistance and rapidly progressed. Using cell-free DNA sequencing and droplet digital PCR of samples taken before and after adagrasib treatment, the researchers identified 10 new mutations in the recurrent tumor, all activating mutations in elements of the MAPK pathway. These included three NRAS mutations, three MAPK mutations, BRAFV600E, and three new KRAS mutations. Of the KRAS mutations, two (KRASG13D and KRASG12V) have already been implicated in resistance. The third, KRASY96D, occurred in a region of KRAS where researchers had not previously identified mutations. Researchers confirmed that adagrasib—which, like other available KRASG12C inhibitors, traps KRAS in an inactive state—could not bind to KRASY96D or inhibit growth of cells expressing the mutant. The researchers then identified a new KRASG12C inhibitor, RM-018, that blocks activity of active-state KRAS, and they showed that the drug was effective against KRASG12C, KRASY96D, and double mutant cells. These data suggest that RM-018 may have therapeutic potential as a new drug to overcome resistance to KRAS inhibitors. This article was featured on the cover of the August issue, and a related commentary can be found here.
Journal: Clinical Cancer Research (August 1 issue)
Oncogenic RET fusions are present in 1 to 2 percent of non-small cell lung cancers (NSCLC), and approximately 50 percent of these cancers will metastasize to the brain. Selpercatinib is a RET inhibitor that was specifically designed to reach sufficient levels in the central nervous system to inhibit RET. Selpercatinib is approved in the United States for the treatment of metastatic NSCLC, but its clinical efficacy for treating brain metastases remains unclear. In this phase I/II clinical trial, researchers evaluated the efficacy of selpercatinib for the treatment of brain metastases in patients with RET fusion-positive NSCLC. Eighty patients with baseline brain metastases were included in the study. Among the 22 patients with measurable intracranial disease at baseline, the intracranial response rate was 82 percent, with 23 percent of patients having complete responses. Among all responders, the median duration of response was not reached after 9.5 months. The median intracranial progression-free survival among all 80 patients was 13.7 months. The authors conclude that selpercatinib may lead to robust and durable intracranial responses in patients with RET fusion-positive metastatic NSCLC. This study was highlighted in the August 1st issue.
Journal: Cancer Prevention Research
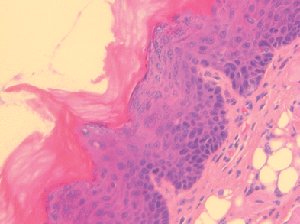
Oral cancer often begins as a patch of atypical cells that progresses to cancer by accumulating additional mutations. Immune checkpoint inhibitors, such as anti-PD-1 antibodies, have been shown to slow the progression of oral premalignant lesions in mouse models, but systemic anti-PD-1 therapy carries the risk of substantial side effects. The authors of this study developed an anti-PD-1 hydrogel that could be locally delivered to oral tumors via injection. The researchers stimulated the development of oral tumors in mice with wild type p53 or R172H mutant p53, using the carcinogen 4-nitroquinolone 1-oxide (4NQO), administered in the drinking water. After four weeks of 4NQO treatment, researchers injected anti-PD-1 hydrogel or control hydrogel into the tongues of the mice and observed the mice for an additional five weeks. Anti-PD-1 hydrogel reduced the number of high-grade tongue lesions from 29 percent to 14 percent in wild type p53 mice and from 60 percent to 20 percent in p53R172H mice, increased the infiltration of CD4+ and CD8+ T cells, and decreased the infiltration of regulatory T cells into the tumors. Tumors often promote immune evasion by silencing the cGAS-STING pathway, which promotes the maturation of antitumor dendritic cells. The researchers showed here that treatment with anti-PD-1 hydrogel restored STING expression and dendritic cell infiltration in wild type p53 tumors, and to a lesser extent in p53R172H tumors. This study suggests that local administration of anti-PD-1 antibodies may delay the progression of premalignant oral lesions into oral cancer. This article was featured on the cover of the August issue.
Journal: Molecular Cancer Research
The majority of glioblastomas contain copy number alteration of the asparagine synthetase (ASNS) gene, an alteration that is associated with reduced survival. In this study, the authors examined the impact of ASNS copy number alteration on brain cancer cells. Using patient-derived glioma stem cells, they found that changes to ASNS gene expression led to metabolic alterations, including a slower basal metabolic profile but with increased capacity for glycolysis and oxidative phosphorylation when needed. These metabolic alterations resulted in increased proliferation and greater spread of glioma stem cells when injected into the brain tissue of mice. Furthermore, changes to metabolism mediated by copy number alteration of ASNS led to radiotherapy resistance due to increased resistance to oxidative stress and other forms of cellular stress. The researchers observed that overexpression of ASNS promoted a tumor microenvironment with increased antioxidant levels, offering a potential therapeutic opportunity. Together, the results suggest that copy number alteration of ASNS leads to metabolic changes that may increase tumor growth and spread and may confer treatment resistance. This article was highlighted in the August issue.
Journal: Cancer Immunology Research
Site-Dependent Immune Escape Due to Impaired Dendritic Cell Cross-Priming

Tumors can evade the immune system in a variety of ways, which can be mediated by the tumor or its microenvironment, and the extent to which the site of tumor origin can predict the immune evasion strategies is currently unclear. In this study, researchers sought to characterize the role of the microenvironment on immune response to pancreatic cancer. Pancreatic cancer cells engineered to express an immunogenic ovalbumin peptide (Ova) were injected into the pancreas, peritoneum, veins, or under the skin of mice and monitored for tumor growth and immune cell infiltration. While cells lacking Ova grew robustly in all sites, cells expressing Ova only grew in the pancreas and peritoneum. Tumors from these sites did not lose expression of Ova or the MHC proteins required to present it, and they did not grow when re-transplanted subcutaneously into new mice, suggesting a site-specific mechanism of immune evasion. The researchers found that mice with pancreatic or peritoneal tumors did not produce as many Ova-targeted T cells as mice injected subcutaneously, and that successful priming of Ova-targeted T cells was dependent upon CD40-mediated dendritic cell activity. Treatment with a CD40 agonist prevented or significantly delayed the growth of pancreatic or peritoneal tumors, while the common immunotherapies anti-PD-1 and anti-CTLA4 had no effect. This study suggests that immune evasion mechanisms are site specific and may require individualized treatment strategies. This article was featured on the cover of the August issue.
Journal: Cancer Research (August 15 issue)
Ultrafine particles (UFP), which are particles less than or equal to 100 nanometers in diameter, can reach the brain through circulation or the olfactory tract and have been suggested to increase the risk of brain cancer. In this study, the authors examined the association between airport-related UFP, which are primarily from aircraft exhaust emissions, and the incidence of brain cancer and meningioma among 75,936 individuals who lived around the Los Angeles International Airport from 1993-1996 through 2013. Among all participants, the risk of brain cancer increased with increasing exposure to airport-related UFP (12 percent increase for every 6,700 particles per cm3). African Americans had the highest exposure to airport-related UFP and had a 32 percent increased risk of brain cancer per interquartile range in UFP exposure. Airport-related UFP was not associated with an increased risk of meningioma. The authors propose that airport-related UFP exposure may be a risk factor for brain cancers.
Journal: Blood Cancer Discovery
Personalized Single-cell Proteomics Distinguish Leukemic and Non-malignant Clones
Clonal hematopoiesis is an aging-related phenomenon in which a hematopoietic stem cell acquires a mutation, which it then passes down to all of its descendants. The mutations observed in clonal hematopoiesis can be similar to those observed in acute myeloid leukemia (AML), making the two conditions difficult to distinguish using bulk DNA sequencing, which can pose problems for AML residual disease monitoring using cell-free DNA technologies. In this study, the researchers used a combination of techniques to distinguish mutant cell populations related to clonal hematopoiesis from those related to AML relapse in three patients. They first performed bulk DNA sequencing on white blood cells to identify mutations potentially involved in either condition. They then created single-cell DNA sequencing probes specific to each patient’s mutations and used a method called CITE-seq, which combines sequencing with mapping of cell-surface proteins to distinguish individual clonal populations. The researchers were able to differentiate AML cells from cells derived from clonal hematopoiesis in all three patients, suggesting that these methods can improve the accuracy of AML relapse monitoring.
Journal: Molecular Cancer Therapeutics
While the 5T4 oncofetal antigen has limited expression in normal adult cells, it is commonly expressed in a variety of malignant tumors, making it a potential therapeutic target. ASN004 is an antibody-drug conjugate designed to deliver cytotoxic auristatin F hydroxypropylamide to 5T4-expressing cells. Here, researchers examined the pharmacology, toxicity, and pharmacokinetic properties of ASN004. They found that ASN004 had high affinity for the 5T4 antigen, and that it selectively bound to, and was internalized by, 5T4-expressing cancer cells. Furthermore, ASN004 demonstrated cytotoxicity against multiple cancer cell lines and led to tumor regression in various tumor xenograft mouse models, including complete regression in a cervical cancer model. Head-to-head comparisons with some existing antibody-drug conjugates showed that ASN004 had greater efficacy than trastuzumab-DM1 and the 5T4-targeted PF-06263507 in gastric tumor and lung tumor models, respectively. ASN004 was also well tolerated in marmosets. The authors conclude that ASN004 may be a promising antibody-drug conjugate and suggest further evaluation in clinical settings. This article was highlighted in the August issue.
Journal: Clinical Cancer Research (August 15 issue)
The genes encoding isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) are mutated in the majority of low-grade gliomas. Vorasidenib is a first-in-class inhibitor of both mutant IDH1 and mutant IDH2 that is able to penetrate the brain. In this phase I clinical trial, researchers evaluated the safety and efficacy of vorasidenib in 93 patients with IDH1/2-mutated solid tumors, including 52 patients with recurrent or progressive gliomas. The researchers report that vorasidenib was associated with a favorable safety profile, and that dose-limiting toxicities were reversible. Eighteen percent of patients with non-enhancing gliomas had an objective response, including one partial response and three minor responses. The median progression-free survival was 36.8 months among patients with non-enhancing gliomas and 3.6 months among those with enhancing gliomas. Additionally, sustained tumor shrinkage was observed in multiple patients with non-enhancing gliomas. The authors conclude that vorasidenib was well tolerated and showed signs of clinical efficacy in patients with recurrent or progressive non-enhancing IDH1/2-mutated gliomas. This article was highlighted in the August 15th issue.
Journal: Cancer Epidemiology, Biomarkers & Prevention
DDT, an insecticide that was used heavily in the mid-20th century, was banned in the U.S. in 1972 amid evidence that it could cause a variety of disorders in humans and animals. Previous studies have shown that the daughters of mothers exposed to DDT while pregnant faced a higher risk of certain cancers, including breast cancer. However, no studies have examined the effects on the granddaughters of exposed women, who were also exposed as oocytes developing in their fetal mothers. The authors of this study measured concentrations of o,p’-DDT—the form of DDT that has been most strongly associated with ill effects in humans—in serum samples from pregnant women collected between 1959 and 1967. From 2010 to 2013, a subset of daughters and granddaughters of these women were invited to participate in the Three Generations of Breast Cancer Study, in which they completed questionnaires and had height, weight, and waist circumference measured during a nurse home visit. The authors of this study found that the granddaughters of normal-weight women in the highest tertile of o,p’-DDT plasma concentrations were two to three times more likely to be obese than the granddaughters of normal-weight women in the lowest tertile of plasma o,p’-DDT and were about twice as likely to experience menarche prior to age 11. As obesity and early menarche both increase breast cancer risk, this study shows that DDT exposures from 60 years ago may still affect cancer risk in women today. This article was highlighted in the August issue, and a related commentary can be found here.
Journal: Cancer Research (August 1 issue)
Gut Microbiota–Derived Short-Chain Fatty Acids Promote Prostate Cancer Growth via IGF1 Signaling
Obesity and consumption of a high-fat diet (HFD) are known to increase the risk of many cancer types, including prostate cancer. Different compositions of gut microbiota have also been shown to influence cancer risk, and as diet heavily influences the microbiome, the authors of this study sought to determine whether antibiotics could inhibit prostate cancer development in HFD-fed mice. Mice genetically engineered to develop prostate tumors were fed a normal diet or HFD, with or without an antibiotic cocktail consisting of ampicillin, vancomycin, metronidazole, neomycin, and gentamicin. HFD-fed mice had a significantly higher prostate tumor burden than mice fed a control diet, and this burden was decreased by antibiotic treatment. Researchers found that the microbiome composition of HFD-fed mice treated with antibiotics was markedly different from the microbiomes of control diet mice or HFD-fed mice that were not given antibiotics. To evaluate the effects of these changes on the prostate, researchers performed gene expression analysis of prostate tissue from HFD-fed mice with or without antibiotic treatment and found 64 differentially regulated genes, including the oncogenic growth factor IGF-1. Further, antibiotic treatment also inhibited the oncogenic MAPK and PI3K pathways, which are known to act downstream of IGF-1. The researchers found that antibiotic-treated mice produced fewer small chain fatty acids, which are known to promote IGF-1 expression, and that re-administration of small chain fatty acids increased prostate IGF-1 expression and prostate tumorigenesis. This study suggests that antibiotic treatment may pose an effective strategy for prostate cancer treatment by decreasing IGF-1 expression. This article was highlighted in the August 1st issue.



