Synergy, Additivity, or Independent Drug Action? A New Study Weighs in on Immunotherapy Combinations
Seven immune checkpoint inhibitors, targeting the PD-1, PD-L1, or CTLA-4 proteins, are currently approved for the treatment of cancer. Unfortunately, these drugs on their own do not elicit clinical responses in the majority of patients, so many of the approved indications for immune checkpoint inhibitors require that they are administered in combination with chemotherapy, targeted therapy, and/or other immunotherapies.
Combining immune checkpoint inhibitors with other cancer therapies has been shown to improve clinical responses for patients with a variety of cancer types, but the underlying reason for the improved efficacy of combinations often remains unclear.
In many cases, it has been assumed that a non-immunotherapeutic cancer drug interacts synergistically with the immune checkpoint inhibitor—that is, its activity enhances the efficacy of the immune checkpoint inhibitor. An alternative, yet less discussed, possibility is that the benefit of combination therapy is due to independent drug action, a concept explained by Peter Sorger, PhD, a professor of systems biology at Harvard Medical School, and colleagues in a recent review article published in the AACR journal Cancer Discovery.

“Whereas a single drug might not be effective in killing every cancer cell in a heterogeneous tumor, drug combinations have the potential to kill different subset of cells, improving the likelihood and durability of response,” they wrote. “The same reasoning also applies to inter-patient heterogeneity: Any single therapy will not be effective in every patient, but combination therapies provide patients with several opportunities for a clinically meaningful response.”
Thus, when a combination works through independent drug action, the benefit to an individual patient is attributed to only one of the drugs in the combination; the benefit over monotherapy is due to “bet hedging,” or increasing the odds that the combination includes a drug that is effective for a given patient.
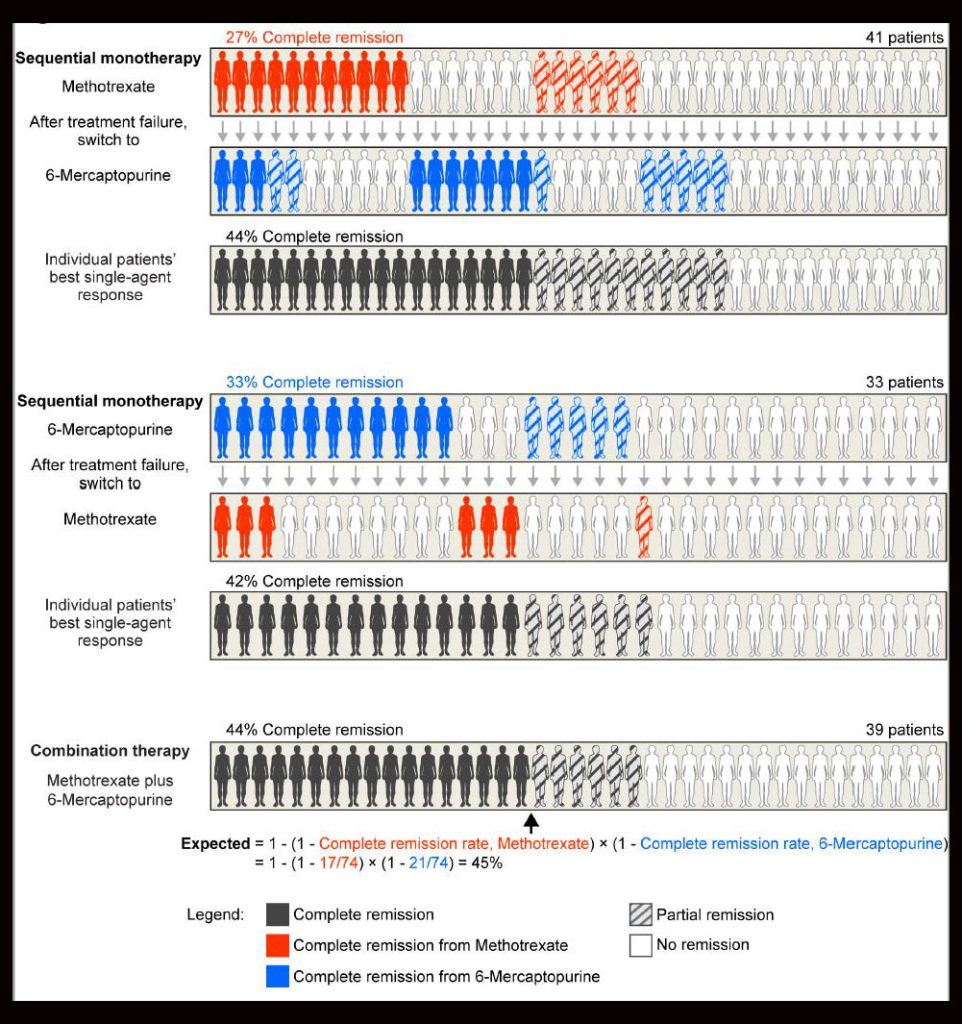
The concept of independent drug action was introduced in 1961 by Emil Frei, MD, FAACR, a pioneer in combination chemotherapy. In a pivotal experiment, Frei and colleagues found that the survival of patients with acute lymphoid leukemia was similar whether they received sequential or simultaneous treatment with 6-mercaptopurine and methotrexate (see figure). The researchers concluded that combining the two drugs did not alter the efficacy of either drug but instead increased the probability of response to one of the drugs.
While independent drug action has been described for decades and has been acknowledged to be the mode of action for many combinations involving chemotherapy or antibiotics, it has not been widely considered as a potential mechanism underlying the efficacy of combinations involving immune checkpoint inhibitors.
To explore the possibility that independent drug action was behind the clinical success of oncology combinations, Sorger and colleagues utilized a predictive model in a retrospective analysis of 13 clinical trials of immune checkpoint inhibitor combination therapies representing eight different cancer types. The results of the analysis were recently published in Clinical Cancer Research, a journal of the AACR.
“For each immunotherapy combination we examined, we used a probability model to calculate the expected progression-free survival distribution that would occur if the combination worked through independent drug action,” explained first author Adam Palmer, PhD, an assistant professor of pharmacology at the University of North Carolina Chapel Hill and a former postdoctoral researcher in Sorger’s lab. The predicted clinical benefit of each drug as monotherapy was based on results from either the same clinical trial as the combination or from another trial with a closely matched cohort that tested the drug as monotherapy. “This expected distribution from independent drug action was then compared to the actual trial result,” Palmer said.

If the actual progression-free survival observed in the clinical trial was not different from the predicted benefit, then the authors concluded that the combination worked through independent drug action; if the actual progression-free survival was significantly greater than the prediction, then the combination benefit was due to synergy or additivity, he added.
Their analyses found that the progression-free survival of patients receiving 12 of the 13 evaluated combinations was similar to or shorter than the predicted outcomes for independent drug action, suggesting that the benefit of these combinations were due to independent drug action rather than synergy or additivity.
In the phase III IMpower150 trial, patients with metastatic non-small cell lung cancer who received first-line treatment with the immune checkpoint inhibitor atezolizumab plus chemotherapy and bevacizumab had longer progression-free survival than would be expected by independent drug action, suggesting that this drug combination may have a synergistic or additive effect on clinical outcomes in this setting.
“Our study revealed that the efficacies of all but one of the combination therapies we analyzed occurred through independent drug action, and not through synergy or additivity,” summarized Palmer. “This is a new finding for immunotherapy combinations, but it’s a very old concept for combination chemotherapy.”
“These findings have important implications for preclinical and clinical research,” said Sorger, suggesting that instead of focusing on achieving drug synergy through interacting mechanisms, researchers should instead combine drugs that are known to have single-agent efficacy in a given disease context. “By combining two drugs that are each effective for some patients with a disease, we can increase the odds that a patient’s cancer will respond to one of the drugs,” he explained.
He added that the study’s findings also indicate a need to better understand variability in treatment response among patients and to identify biomarkers that could accurately predict a patient’s response to individual drugs in a combination.
“What we really need…is not necessarily new drugs. We need more precision,” Sorger said. “We need to get drugs into the right populations of patients. The criticism of [our] work is that what we’re saying is you can just combine any old drugs without knowing any mechanism, and that’s definitively not the case.” For immunotherapy combinations working by independent drug action, greater precision could yield substantial improvements in clinical outcomes even with existing drugs, a notion supported by preclinical research, he added.
Furthermore, the propensity of independent drug action in combination therapies suggests that administering drugs sequentially, rather than together, might reduce toxicities without impacting clinical efficacy. However, Palmer noted that this may not always be possible in patients with rapidly progressing cancer.
“To be clear, we are not suggesting that these combinations are ineffective. We agree that the combinations are clinically effective; what we are proposing is that their effectiveness is through a different mechanism than was previously thought,” said Sorger. “These are classical and well established ideas. They just haven’t been testable [until now].”