AACR Journal Editors Share Featured Articles
For the first Editors’ Picks collection of 2022, the editors of the AACR journals highlighted studies that assessed the impact of work-related stress on cancer risk, a mechanism through which chemotherapy can promote metastasis, a new-generation CAR T-cell therapy for acute myeloid leukemia, and more. Enjoy reading the featured articles, which will be freely available online for a limited time.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Stress has been suggested to be a risk factor for cancer, but the impacts of stress related to work are less understood. In this study, researchers assessed how work-related stress affected cancer incidence in a population-based cohort of 113,057 individuals using data from Swedish national registries. Work-related stress was assessed using the Karasek job demand/control model, and results were adjusted for potential confounding variables. The analyses did not find any significant differences in cancer risk between high-strain work and low-strain work. Most cancer type-specific analyses also showed no significant associations between work-related stress and cancer risk. Together, the results suggest that work-related stress may not be a significant risk factor for cancer. This article was highlighted in the January issue.
Journal: Molecular Cancer Therapeutics
Protein arginine methyltransferase 5 (PRMT5) catalyzes the methylation of arginine residues in proteins involved in a variety of cellular processes, acting as a master regulator of growth and development. PRMT5 is overexpressed in many cancer types, including non-small cell lung cancer (NSCLC), and is associated with increased tumor growth and reduced patient survival. Therefore, PRMT5 inhibition is an attractive anticancer pharmacologic approach, and a few compounds are currently in clinical trials. This study led to the identification of PF-06939999, a potent and selective S-adenosylmethionine (SAM) competitive PRMT5 inhibitor that reduced the proliferation of NSCLC cancer cells, with dose-dependent decreases in enzyme activity. The authors developed drug-resistant cell lines to dissect the potential mechanisms of resistance to PF-06939999 and identified structural constraint that make SAM-competitive PRMT5 inhibitors less likely than other inhibitors to induce PRTM5 mutations that confer complete drug resistance. Since PRMT5 regulates the activity of splicing factors, PRMT5 inhibition is known to be associated with reduced splicing fidelity and selective killing of leukemias carrying spicing factor mutations. In this study, PF-06939999 treatment of NSCLC cells resulted in alternative splicing changes, both exon skipping and intron retention events. Mutations in RBM10, which is the splicing factor most affected by mutation in NSCLC, sensitized the cells to the inhibitor. According to the authors, these findings support the clinical development of PF-06939999 for the treatment of NSCLC with dysregulated splicing. This article was featured on the cover and highlighted in the January issue.
Journal: Cancer Research (January 1 issue)
Many non-small cell lung cancers (NSCLC) have mutations in the epidermal growth factor receptor (EGFR), and the standard treatment for such tumors is an EGFR inhibitor. However, patients frequently develop resistance to EGFR inhibitors, necessitating new strategies to improve their efficacy. Researchers have previously shown that HER3, a related receptor, is highly expressed in EGFR-mutant NSCLCs, leading to the hypothesis that a HER3-targeted antibody-drug conjugate may be effective against these tumors. In this study, the researchers examined the efficacy of HER3-DXd—which consists of a topoisomerase inhibitor conjugated to a HER3-targeting antibody—in preclinical models of EGFR inhibitor-resistant NSCLC. They found that HER3-Dxd treatment led to growth delay or tumor regression in EGFR inhibitor-resistant patient-derived xenograft models. In both EGFR inhibitor-naïve and -resistant cell lines, the EGFR inhibitor osimertinib (Tagrisso) increased expression, membrane localization, and internalization of HER3, suggesting that osimertinib pretreatment may prime cells for a better response to HER3-DXd. Indeed, cells and PDX tumor fragments pretreated for 24 hours with osimertinib and then treated for several days with osimertinib plus HER3-DXd experienced significantly delayed growth and increased apoptosis as compared with either treatment alone. The researchers suggested that combining osimertinib with HER3-DXd may improve patient response and potentially delay or prevent resistance. This study was featured on the cover of the January 1 issue, and a related commentary can be found here.
Journal: Clinical Cancer Research (January 15 issue)
Advanced soft tissue sarcomas are typically treated with palliative chemotherapy. Due to the increased expression and activity of the MET and VEGF proteins in sarcoma cell lines and patient tumors, respectively, the authors of this study hypothesized that dual targeting of the MET and VEGF pathways with cabozantinib may be an effective treatment against this cancer. To test their hypothesis, the authors conducted a phase II clinical trial that evaluated single-agent cabozantinib in adult patients with advanced soft tissue sarcomas that had progressed after prior systemic therapy. Among the 54 evaluable patients, six had partial responses to the treatment with a median duration of response of 16 months. Six-month progression-free survival was observed in 49.3 percent of patients. The most common grade 3/4 adverse events were hypertension and neutropenia. The trial did not meet its primary endpoints. This article was highlighted in the January 15 issue.
Journal: Cancer Research (January 15 issue)
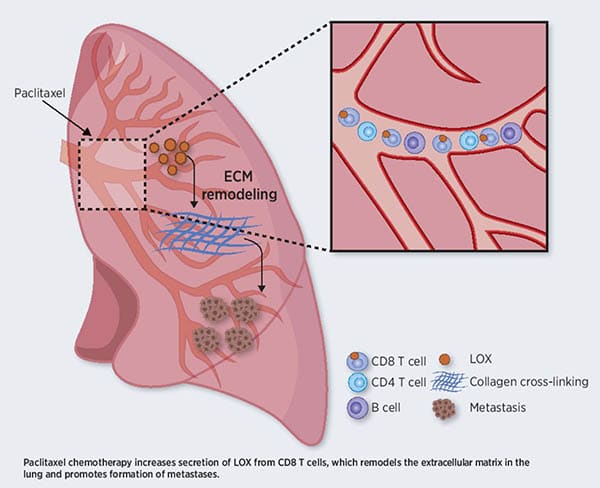
T Cells Promote Metastasis by Regulating Extracellular Matrix Remodeling following Chemotherapy
Chemotherapy remains one of the most potent tools available for cancer treatment. However, increasing evidence suggests that, in some cases, chemotherapy can result in pro-tumorigenic and pro-metastasis effects through host-mediated mechanisms that are not fully understood. In this study, the authors found that the chemotherapy drug paclitaxel promoted remodeling of the extracellular matrix (ECM) in the lungs of tumor-free mice by increasing the levels and activity of the lysyl oxidase (LOX) enzyme, which cross-links collagen and elastin and is a key mediator of tumor growth and metastasis. Indeed, the ECM changes observed following paclitaxel treatment facilitated cancer cell spreading in vitro and in vivo. The paclitaxel-mediated ECM remodeling occurred in a systemic fashion, as plasma from paclitaxel-treated mice promoted ECM remodeling in the lungs of receiving mice. A chimeric mouse model with genetic LOX depletion in the bone marrow revealed that this effect was mediated by CD8+ T cells expressing LOX, and transfer of CD8+ T cells, but not other immune cells, from paclitaxel-treated mice to naïve immunodeficient mice induced ECM remodeling in the lungs. Furthermore, in a clinically relevant mouse model of metastatic breast carcinoma, treatment with a LOX inhibitor counteracted the metastasis-promoting effects of paclitaxel mediated by ECM remodeling. This study highlighted the role of CD8+ T cells in priming the lung microenvironment for metastatic spreading following chemotherapy. According to the authors, these findings suggest that LOX may be targeted to prevent these ECM remodeling effects of chemotherapy and enhance its efficacy in the treatment of metastatic cancer. This article was featured on the cover of the January issue and highlighted in a commentary.
Journal: Blood Cancer Discovery
NOT-Gated CD93 CAR T Cells Effectively Target AML with Minimized Endothelial Cross-Reactivity
While chimeric antigen receptor (CAR) T-cell therapy has been beneficial for some patients with B-cell acute lymphoblastic leukemia or diffuse large B-cell lymphoma, CAR T-cell therapies have thus far resulted in severe toxicity and limited efficacy in patients with acute myeloid leukemia (AML). In this study, researchers developed a second-generation CAR T cell that targeted CD93, a cell surface protein expressed in many AML cases with limited expression in nonhematopoietic tissues. In preclinical experiments, the CD93-targeted CAR T-cell therapy led to leukemic clearance with minimal toxicities. However, the CD93 CAR T cells targeted CD93-expressing endothelial cell lines, which the researchers found could be mitigated by employing a NOT-gate strategy. This strategy employs an inhibitory CAR that is specific for an antigen present on healthy, but not cancerous, cells; if the CAR T cell binds the antigen on a healthy cell, the cytotoxic activity of the CAR T cell is inhibited, thereby preventing targeting of non-cancerous cells. The authors also performed transcriptome profiling on AML cells and endothelial cells to identify additional candidate targets for CAR T-cell therapy. The authors conclude that NOT-gated CD93 CAR T-cell therapy targeted AML while minimizing off-target reactivity with endothelial cells in preclinical models. This article was highlighted in the January issue. A related commentary can be found here.
Journal: Cancer Immunology Research
Imaging α-GalCer–Activated iNKT Cells in a Hepatic Metastatic Environment
Liver metastases commonly develop in patients with colorectal cancer, potentially due to surgical resection of the primary tumor, which may release tumor cells into circulation. Immune cells in the liver microenvironment may help prevent tumor cell seeding in the liver. Here, the authors used an in vivo imaging model that allowed them to track fluorescently labeled tumor cells in living mice. With this model, they examined how activation of invariant natural killer T (iNKT) cells in the liver microenvironment affected metastasis to the liver. They found that prophylactic stimulation of iNKT cells with multiple doses of the immunomodulatory drug α-galactosylceramide (α-GalCer) reduced tumor growth in the liver. The authors propose that early administration of α-GalCer prior to surgical resection of the primary colorectal tumor could be an effective strategy to reduce liver metastases in patients. This article was featured on the cover of the January issue.
Journal: Cancer Discovery
Fasting-mimicking Diet is Safe and Reshapes Metabolism and Antitumor Immunity in Cancer Patients
In preclinical models, severe calorie restriction was associated with potent anticancer effects when combined with pharmacological treatments. This clinical study evaluated the safety and biological effects of calorie restriction in the form of a fasting-mimicking diet (FMD) in 101 cancer patients with various tumor types treated with different standard anticancer therapies. The regimen consisted of a five-day, low-carbohydrate, low-protein, plant-derived diet and was repeated every three or four weeks for up to eight consecutive times. With a high compliance rate and an incidence of severe FMD-related adverse events of 12.9 percent, this study demonstrated that short-term severe calorie restriction was safe, feasible, and well tolerated. The loss of body weight observed after a five-day cycle was reversible in most of the patients during the refeeding period. The researchers also observed FMD-induced metabolic changes, including reduced median plasma glucose concentration, serum insulin, and serum IGF-1, which remained stable over the course of eight consecutive cycles. Furthermore, an analysis conducted on a subset of participants at the end of an FMD cycle revealed a significant decrease of circulating immunosuppressive myeloid subpopulations and an increase of activated CD8+ T cells. The study also included an interim analysis of another trial testing a five-day FMD cycle before surgery in early-stage breast cancer and melanoma patients, which revealed a significant increase in tumor-infiltrating CD8+ T cells and other changes suggestive of a functional switch toward an antitumor immune microenvironment following FMD. This article was highlighted in the January issue and featured in an AACR press release.
Journal: Clinical Cancer Research (January 1 issue)
For patients with ovarian cancer who develop resistance to platinum-based chemotherapy, response to subsequent chemotherapy is often limited. The safety and efficacy of the DNA damage checkpoint inhibitor adavosertib has been studied in patients with solid tumors, both alone and in combination with chemotherapy. Because ovarian tumors have a high prevalence of mutations in DNA repair pathway genes, such as p53, adavosertib may boost the efficacy of chemotherapy in patients with platinum-resistant disease. In this phase II clinical trial, researchers enrolled 94 patients with platinum-resistant ovarian cancer and randomly assigned them to one of six treatment groups: adavosertib plus gemcitabine, adavosertib plus paclitaxel, two different adavosertib dosing regimens plus carboplatin, and two different adavosertib doses plus pegylated liposomal doxorubicin (PLD). Patients treated with adavosertib twice daily for three consecutive days each week plus carboplatin every 21 days had the best overall response rate of 66.7 percent, the best disease control rate of 100 percent, and a median progression-free survival of 12 months. Genotyping of tumor tissue revealed that adavosertib plus carboplatin was effective in patients with and without p53-mutated tumors. While the researchers cautioned that combination therapy resulted in a higher rate of hematological toxicity than observed in previous trials of carboplatin alone, they suggested that further studies could position adavosertib plus carboplatin as a promising ovarian cancer treatment. This article was highlighted in the January 1 issue.
Journal: Molecular Cancer Research
The epithelial-mesenchymal transition (EMT) is known to drive metastasis in many solid tumor types, and it is regulated by epigenetic drivers such as lysine-specific demethylase 1 (LSD1). Malignant pleural mesothelioma is an aggressive cancer with few effective treatment options, and tumors often contain clusters of mesenchymal-like cells that correlate with poor prognosis. The authors of this study sought to determine whether inhibiting EMT using genetic or pharmacologic LSD1 blockade could decrease survival of malignant pleural mesothelioma cells. Abrogation of LSD1 expression in sarcomatoid cells—a more mesenchymal subtype of malignant pleural mesothelioma—mediated a switch to a more epithelial phenotype, made cells more sensitive to cisplatin chemotherapy, and suppressed activation of focal adhesion kinase (FAK). Genetic analyses of LSD1-deficient cells revealed that LSD1 regulates expression of milk fat globulin E8 (MFGE8), which is known to stimulate FAK activity. The researchers showed that LSD1 promotes expression of MFGE8, which activates FAK by binding to the αvβ3 integrin, in turn driving expression of the master EMT regulator Snail. High expression of MFGE8 in serum from malignant pleural mesothelioma patients correlated with significantly shorter overall survival, and addition of exogenous MFGE8 protein to LSD1-deficient cells decreased their cisplatin sensitivity. These data present a novel mechanism underlying EMT in malignant pleural mesothelioma and position LSD1 blockade as a potential therapeutic strategy. This article was highlighted in the January issue.
Journal: Cancer Prevention Research
Most colon cancers develop from intestinal crypts—pockets of stem cells that replenish healthy intestinal tissue every three to five days. Early colon tumorigenesis can be driven by a decrease in aryl hydrocarbon receptor (AHR) signaling, which can disrupt the normal relationships between stem cells and progenitor cells, but the mechanism remains unknown. In this study, the researchers performed single-cell RNA sequencing on mouse intestinal crypts with stem cells lacking AHR expression (AHR knockout) as well as crypts with normal AHR expression (AHR wild type). They found that cells from AHR knockout crypts had lower expression of genes involved in cholesterol homeostasis, oxidative phosphorylation, and p53 signaling. The researchers also measured the cell differentiation potency—a measure of cell stemness—of all cell types within the crypts and found that both stem and progenitor cells from AHR knockout crypts had a higher differentiation potency than cells from AHR wild type crypts. Another measurement of differentiation potential, called RNA velocity, can show researchers how quickly stem cells are differentiating, and AHR knockout cells had significantly higher RNA velocities than AHR wild type cells. This study highlights the importance of AHR in maintaining normal intestinal stem cell function, and the authors suggest AHR stimulation could become an important colon cancer prevention strategy. This study was featured on the cover of the January issue.